Enhancing Luminescence Performance of Te-Substituted Cs2SnCl6 through Te–Te Distance Tuning and Strain Optimization
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
To achieve highly efficient luminescence with A2BX6-type tin(IV)-based metal halide perovskites, it is essential to overcome their parity-forbidden electronic transition characteristics. For Cs2SnCl6, Te substitution has been demonstrated as an effective strategy; however, its luminescence can be significantly reduced or even quenched under certain conditions. To uncover the microscopic mechanisms driving this behavior and optimize the luminescence performance of Te-substituted Cs2SnCl6, we conducted first-principles calculations to examine the effects of the Te–Te distance and strain conditions. Our findings reveal that the cosubstitution of Te atoms at two nearest-neighbor Sn positions induces significant octahedral distortion, energy band broadening, and an increase in the transition dipole moment, which collectively enhance luminescence performance. However, at high Te substitution concentrations, electron transitions are suppressed, resulting in luminescence quenching. In terms of mechanical strain, we found that uniaxial tensile strain improves luminescence performance, while uniaxial compressive strain and biaxial strain have a detrimental effect. These findings provide valuable insights into the luminescence mechanism of Te-substituted Cs2SnCl6 and may be applicable to other related tin halide perovskite materials, offering important guidance to optimize their luminescence properties for advanced optoelectronic applications.
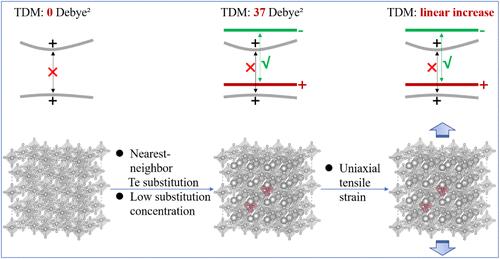
通过Te-Te距离调谐和应变优化提高te取代Cs2SnCl6的发光性能
为了实现a2bx6型锡基金属卤化物钙钛矿的高效发光,必须克服其奇偶禁止电子跃迁特性。对于Cs2SnCl6,取代被证明是一种有效的策略;然而,在一定条件下,它的发光会明显减弱甚至猝灭。为了揭示驱动这种行为的微观机制并优化te取代Cs2SnCl6的发光性能,我们进行了第一性原理计算来研究Te-Te距离和应变条件的影响。我们的研究结果表明,Te原子在两个相邻Sn位置的共取代引起了显著的八面体畸变、能带拓宽和跃迁偶极矩的增加,这些共同增强了发光性能。然而,在高Te取代浓度下,电子跃迁被抑制,导致发光猝灭。在机械应变方面,我们发现单轴拉伸应变提高了发光性能,而单轴压缩应变和双轴应变对发光性能有不利影响。这些发现为te取代Cs2SnCl6的发光机理提供了有价值的见解,并可能适用于其他相关的卤化锡钙钛矿材料,为优化其发光性能提供重要指导,用于先进的光电应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


