GIPR-Ab/GLP-1 peptide–antibody conjugate requires brain GIPR and GLP-1R for additive weight loss in obese mice
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
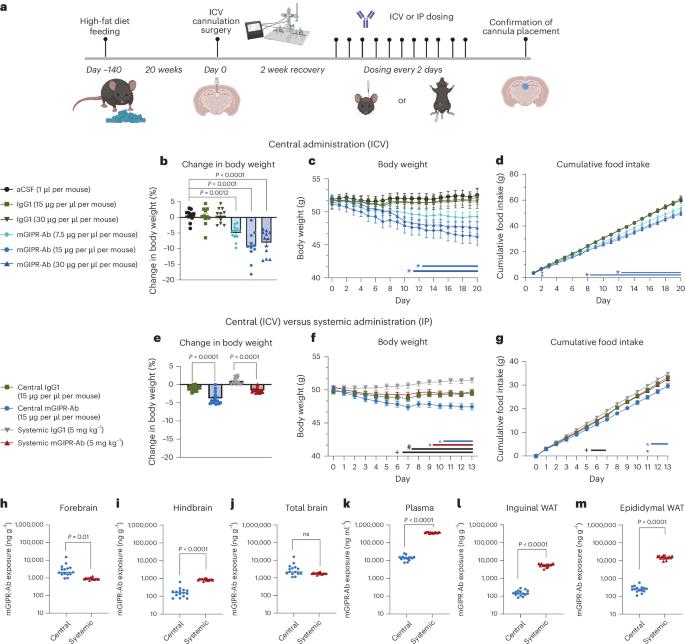
Glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide 1 receptor (GLP-1R) are expressed in the central nervous system (CNS) and regulate food intake. Here, we demonstrate that a peptide–antibody conjugate that blocks GIPR while simultaneously activating GLP-1R (GIPR-Ab/GLP-1) requires both CNS GIPR and CNS GLP-1R for maximal weight loss in obese, primarily male, mice. Moreover, dulaglutide produces greater weight loss in CNS GIPR knockout (KO) mice, and the weight loss achieved with dulaglutide + GIPR-Ab is attenuated in CNS GIPR KO mice. Wild-type mice treated with GIPR-Ab/GLP-1 and CNS GIPR KO mice exhibit similar changes in gene expression related to tissue remodelling, lipid metabolism and inflammation in white adipose tissue and liver. Moreover, GIPR-Ab/GLP-1 is detected in circumventricular organs in the brain and activates c-FOS in downstream neural substrates involved in appetite regulation. Hence, both CNS GIPR and GLP-1R signalling are required for the full weight loss effect of a GIPR-Ab/GLP-1 peptide–antibody conjugate. This study, together with a companion manuscript, shows that in mice, weight loss as a result of GIP receptor antagonism requires and potentiates functional GLP-1 receptor signalling in the brain, explaining how both GIP receptor agonists and antagonists trigger weight loss through different mechanisms.

GIPR- ab /GLP-1肽抗体偶联物需要脑GIPR和GLP-1R来实现肥胖小鼠的增重
葡萄糖依赖性胰岛素性多肽受体(GIPR)和胰高血糖素样肽1受体(GLP-1R)在中枢神经系统(CNS)中表达并调节食物摄入。在这里,我们证明了阻断GIPR同时激活GLP-1R的肽抗体偶联物(GIPR- ab /GLP-1)需要CNS GIPR和CNS GLP-1R才能在肥胖小鼠(主要是雄性小鼠)中实现最大的体重减轻。此外,杜拉鲁肽在CNS GIPR基因敲除(KO)小鼠中产生更大的体重减轻,而杜拉鲁肽+ GIPR- ab在CNS GIPR基因敲除(KO)小鼠中的体重减轻效果减弱。GIPR- ab /GLP-1野生型小鼠和CNS GIPR KO小鼠在白色脂肪组织和肝脏组织重塑、脂质代谢和炎症相关基因表达方面表现出相似的变化。此外,在脑的心室周围器官中检测到GIPR-Ab/GLP-1,并激活参与食欲调节的下游神经底物中的c-FOS。因此,中枢神经系统的GIPR和GLP-1R信号都是GIPR- ab /GLP-1肽-抗体偶联物完全减重所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


