Multicomponent synthesis of unsymmetrical 1,2-diamines via photo-induced carbonyl alkylative amination†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00479a
引用次数: 0
Abstract
Vicinal diamines (or 1,2-diamines) are privileged structural motifs present in many bioactive molecules and drug candidates. The past few decades have witnessed substantial progress in the synthesis of unsymmetrical 1,2-diamines via several strategies, including olefin diamination and α-amino carbanion- or radical-mediated Mannich reactions. However, methods for the one-step preparation of valuable N,N′-dialkylated 1,2-diamines are still rarely reported. We report herein a photo-induced carbonyl alkylative amination reaction that, for the first time, brings together amines, aldehydes, and iodoarenes under catalyst- and base-free conditions for the synthesis of diverse alkyl/aryl-substituted unsymmetrical 1,2-diamines. This method shows a broad scope and good functional group tolerance. Furthermore, detailed mechanistic investigations reveal that the reaction proceeds via a visible light-mediated radical chain process.
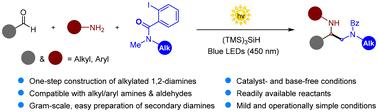
光诱导羰基烷基化胺化多组分合成不对称1,2-二胺
邻二胺(或1,2-二胺)是存在于许多生物活性分子和候选药物中的特殊结构基序。在过去的几十年里,通过烯烃二化、α-氨基碳离子或自由基介导的曼尼希反应等多种方法合成不对称1,2-二胺的研究取得了长足的进展。然而,一步法制备有价N,N ' -二烷基化1,2-二胺的方法仍然很少报道。本文报道了一种光诱导羰基烷基化胺化反应,首次在无催化剂和无碱条件下,将胺、醛和碘芳烃聚集在一起,合成了多种烷基/芳基取代的不对称1,2-二胺。该方法适用范围广,对官能团具有良好的容忍度。此外,详细的机理研究表明,该反应是通过可见光介导的自由基链过程进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


