Electrochemical Oxidation of Methane to Methylamine
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical methane (CH4) oxidation reaction under ambient conditions is a promising approach for natural gas utilization. However, the highly stable nature of CH4 restrains efficient cleavage of the C–H bond without overoxidation. To this end, we proposed a coupled oxidative strategy for converting CH4 to value-added products via NH2 intermediates. The ammonia-oxidation-driven anodic reaction facilitates the formation of NH2 species and activates CH4 through nucleophilic attack to achieve C–N bond formation. Further the generated methylamine could be protected from overoxidation in the ammonia-based anolyte. Under such conditions, a methylamine productivity of 3.8 mmol g–1cat h–1 at 1.55 V vs a reversible hydrogen electrode is reported. This work provides new insights into electrocatalytic C–N coupling based on the ammonia oxidation reaction, which could extend to other alkane or alkene conversions.
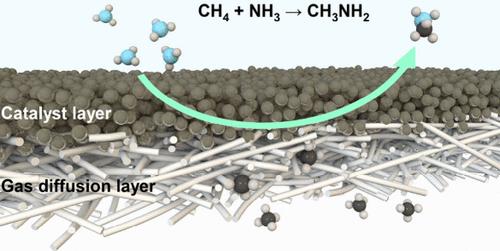
电化学氧化甲烷生成甲胺
环境条件下的电化学甲烷(CH4)氧化反应是一种很有前景的天然气利用方法。然而,CH4 的高稳定性限制了在不过度氧化的情况下有效裂解 C-H 键。为此,我们提出了一种通过 NH2 中间体将 CH4 转化为高附加值产品的耦合氧化策略。氨氧化驱动的阳极反应促进了 NH2 物种的形成,并通过亲核攻击激活了 CH4,实现了 C-N 键的形成。此外,生成的甲胺还能在氨基电解质中免受过氧化反应的影响。在这种条件下,与可逆氢电极相比,在 1.55 V 电压下,甲胺的生产率为 3.8 mmol g-1cat h-1。这项工作为基于氨氧化反应的电催化 C-N 偶联提供了新的见解,并可扩展到其他烷烃或烯的转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


