Green and modular synthesis of azepino[4,3,2-cd]indoles and diazepino[6,5,4-cd]indoles via hydride transfer-involved cascade cyclization in ethanol†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00058k
引用次数: 0
Abstract
The modular synthesis of high-value azepino[4,3,2-cd]indole and its analogs from versatile synthons and readily available feedstocks has been a challenging area of research. Herein, we report a 1,6-hydride transfer-involved cascade [6 + 1] cyclization of 4-dialkylamino-indole-3-carbaldehydes with diverse 1C,1C- and 1N,1N-bisnucleophiles in ethanol, enabling the green and modular synthesis of azepino[4,3,2-cd]indoles and diazepino[6,5,4-cd]indoles. The key features of this method are: catalyst-free, redox-neutral conditions, excellent substrate compatibility, operational simplicity, gram-scale applicability, and versatile product derivatization.
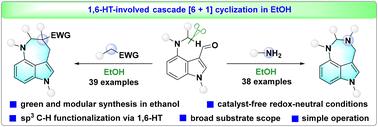
乙醇中氢化物转移级联环化绿色模块化合成氮平[4,3,2-cd]吲哚和二氮平[6,5,4-cd]吲哚
利用多种多样的合成物和现成的原料,模块化合成高价值的氮杂吲哚[4,3,2-cd]及其类似物一直是一个具有挑战性的项目。在本文中,我们报道了1,6-氢化物转移参与级联[6 + 1]环化4-二烷基氨基-吲哚-3-乙醛与不同的1C,1C-和1N,1N -双亲核试剂在乙醇中,实现了绿色和模块化合成二氮平[6,5,4-cd]吲哚和二氮平[6,5,4-cd]吲哚。该方法具有无催化剂氧化还原中性条件,良好的底物相容性,简单的操作程序,克级合成和多样化的产品衍生性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


