Electrochemical carboxylation of α-fluoroalkyl cyclopropane with CO2 to mono- or difluoropentenoic acid†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00223k
引用次数: 0
Abstract
An electrochemical carboxylation of α-fluoroalkyl cyclopropanes with CO2 is reported in this work. This approach constitutes a rare example of defluorinative carboxylation of organofluorine compounds with the simultaneous cleavage of C–F and C–C bonds. Accordingly, both α-CF2H and α-CF3 cyclopropanes serve as effective substrates, facilitating the synthesis of pentenoic acids with an E-configured monofluoroalkene or gem-difluoroalkene moiety with high chemo- and stereoselectivity. The reaction can be also performed under a nonsacrificial anode system. The synthetic practicality is further highlighted by the diverse functionalizations of the resulting multifunctional fluorinated acids. Cyclic voltammetry studies were performed to provide mechanistic insights into the reaction's origins.
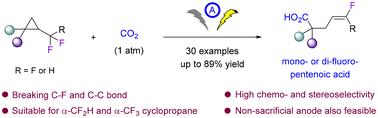
α-氟烷基环丙烷与CO2的电化学羧化反应制单氟或二氟戊烯酸
本文报道了α-氟烷基环丙烷与CO2的电化学羧基化反应。这种方法构成了有机氟化合物的脱氟羧基化与同时切割C-F和C-C键的罕见例子。因此,α-CF2H和α-CF3环丙烷作为有效底物,有利于合成具有高化学选择性和立体选择性的e构型单氟烯烃或双氟烯烃片段的戊烯酸。该反应也可以在非牺牲阳极体系下进行。合成的实用性进一步突出了所得到的多功能氟化酸的不同功能化。进行循环伏安法研究,以提供对反应起源的机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


