Intermediate States Enable Keratin-like α-To-β Transformations in Strain-Responsive Synthetic Polypeptides
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
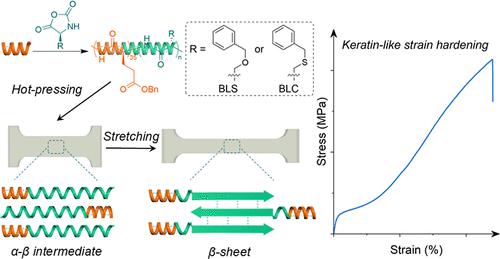
Nature’s fibrous proteins, such as α-keratin, achieve remarkable mechanical properties by undergoing strain-induced α-to-β conformational transitions. Inspired by these materials, we report a strategy for designing synthetic polypeptides that undergo similar transformations at elevated temperatures far exceeding keratin’s operational range. By employing helix-confined ring-opening polymerization (ROP) of N-carboxyanhydrides (NCAs) initiated by a short poly(γ-benzyl-l-glutamate) (PBLG) precursor, we synthesized poly(O-benzyl-l-serine) (PBLS) chains that adopt an α-helical structure yet transition into β-sheets upon heating. Compression molding at carefully chosen temperatures drives PBLS segments into an α-β intermediate state, characterized by relaxed intrachain hydrogen bonds and a hexagonal packing arrangement. Under mechanical strain, these intermediate states convert in situ into β-sheets, producing significant strain-hardening well below the spontaneous α-to-β temperature threshold. This approach extends to polypeptides bearing different side chains, such as poly(S-benzyl-l-cysteine), demonstrating robust mechanical reinforcement across a wide temperature window up to ∼ 200 °C. In situ synchrotron X-ray analysis confirms that chain alignment, β-sheet formation, and domain growth occur stepwise during deformation. By harnessing the intermediate states and the supramolecular cooperativity conferred by compression-molded films, our method provides a versatile platform for developing next-generation polypeptide materials with tunable mechanical resilience and responsiveness─surpassing the temperature limitations of natural fibrous proteins and enabling potential applications demanding broad-temperature mechanical adaptability.
在菌株响应的合成多肽中,中间状态使角蛋白样α-To-β转化成为可能
自然界的纤维蛋白,如α-角蛋白,通过应变诱导的α -β构象转变获得了显著的机械性能。受这些材料的启发,我们报告了一种设计合成多肽的策略,这些多肽在远远超过角蛋白的操作范围的高温下进行类似的转化。采用短聚γ-苄基-l-谷氨酸(PBLG)前体引发的n -羧基氢化物(NCAs)的螺旋约束开环聚合(ROP),合成了α-螺旋结构的聚(o -苄基-l-丝氨酸)(PBLS)链,该链加热后转变为β-片。在精心选择的温度下进行压缩成型,可使PBLS片段进入α-β中间状态,其特征是链内氢键松弛和六边形填充排列。在机械应变作用下,这些中间态就地转化为β-薄片,产生明显的应变硬化,远低于自发α -β温度阈值。这种方法扩展到具有不同侧链的多肽,例如聚(s -苄基-l-半胱氨酸),在高达200°C的宽温度窗口内显示出强大的机械增强。原位同步加速器x射线分析证实,在变形过程中,链排列、β-薄片形成和畴生长是逐步发生的。通过利用中间状态和压缩成型薄膜所赋予的超分子协同性,我们的方法为开发具有可调机械弹性和响应性的下一代多肽材料提供了一个通用平台──超越了天然纤维蛋白的温度限制,并实现了需要宽温度机械适应性的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


