ZBP1 senses spliceosome stress through Z-RNA:DNA hybrid recognition
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Z-DNA-binding protein 1 (ZBP1; also known as DAI or DLM-1) regulates cell death and inflammation by sensing left-handed double-helical nucleic acids, including Z-RNA and Z-DNA. However, the physiological conditions that generate Z-form nucleic acids (Z-NAs) and activate ZBP1-dependent signaling pathways remain largely elusive. In this study, we developed a probe, Zα-mFc, that specifically detected both Z-DNA and Z-RNA. Utilizing this probe, we discovered that inhibiting spliceosome causes nuclear accumulation of Z-RNA:DNA hybrids, which are sensed by ZBP1 via its Zα domains, triggering apoptosis and necroptosis in mammalian cells. Furthermore, we solved crystal structures of the human or mouse Zα1 domain complexed with a 6-bp RNA:DNA hybrid, revealing that the RNA:DNA hybrid adopts a left-handed conformation. Our findings demonstrate that the spliceosome acts as a checkpoint preventing accumulation of Z-RNA:DNA hybrids, which potentially function as endogenous ligands activating ZBP1-dependent cell death pathways.
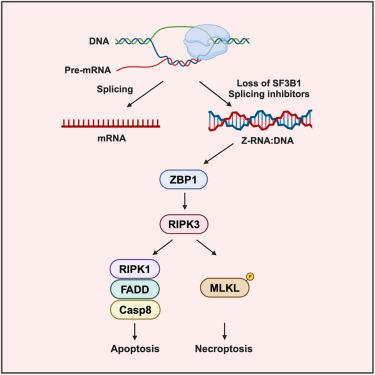
ZBP1通过Z-RNA:DNA杂交识别来感知剪接体的应激
z - dna结合蛋白1;也被称为DAI或DLM-1)通过感应左旋双螺旋核酸(包括Z-RNA和Z-DNA)调节细胞死亡和炎症。然而,产生z型核酸(Z-NAs)和激活zbp1依赖的信号通路的生理条件在很大程度上仍然难以捉摸。在这项研究中,我们开发了一种探针,Zα-mFc,特异性检测Z-DNA和Z-RNA。利用该探针,我们发现抑制剪接体会引起Z-RNA:DNA杂合体的核积累,ZBP1通过其Zα结构域感知这些杂合体,从而引发哺乳动物细胞的凋亡和坏死。此外,我们求解了人类或小鼠Zα1结构域与6 bp RNA:DNA杂合物的晶体结构,发现RNA:DNA杂合物采用左旋构象。我们的研究结果表明,剪接体作为一个检查点阻止Z-RNA:DNA杂交体的积累,这可能作为内源性配体激活zbp1依赖的细胞死亡途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


