Enantioselective Synthesis of Inherently Chiral Tetraazacalix[4]aromatics from a Chiral Phosphoric Acid-Catalyzed Intramolecular SNAr Reaction
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Inherently chiral calixarenes and heteracalixaromatics (ICCHeC) have been drawing great attention, because of their unique 3D stereochemical structures and tantalizing applications. The majority of ICCHeC arises from the cyclic arrays of different aromatic segments. Examples of ICCHeC generated from various substituents on methylene and heteroatom linkages are extremely rare. Here, we report the enantioselective synthesis of tetraazacalix[1]arene[1]pyridine[2]triazines from a chiral phosphoric acid-catalyzed SNAr reaction. The inherent chirality of the resulting 1,3-alternate heteracalix[4]aromatics stems from the variation of only one substituent on the bridging nitrogen atom. Enantiomers were very stable, and they did not undergo racemization at an elevated temperature. This study opens a new avenue to design and construct novel and functional ICCHeC that may find useful applications in supramolecular science.
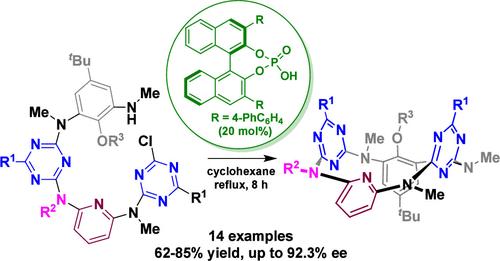
手性磷酸催化的分子内SNAr反应对映选择性合成固有手性四氮杂环[4]芳烃
固有手性杯芳烃和杂杯芳烃(ICCHeC)因其独特的三维立体化学结构和诱人的应用前景而备受关注。大多数ICCHeC是由不同芳香段的环状排列产生的。由亚甲基和杂原子键上的各种取代基生成的ICCHeC的例子非常罕见。本文报道了用手性磷酸催化SNAr反应对映选择性合成四氮杂环[1]芳烃[1]吡啶[2]三嗪。所得到的1,3-交联杂臂[4]芳烃的固有手性源于桥接氮原子上仅一个取代基的变化。对映体非常稳定,在高温下不会发生外消旋。本研究为设计和构建新型功能性ICCHeC开辟了一条新的途径,并可能在超分子科学中找到有用的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


