Enantioselective Formation of Isoxazolidines via Brønsted-Base Catalyzed (3 + 2) Annulation of Cyclopropanes with Nitrosoarenes
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
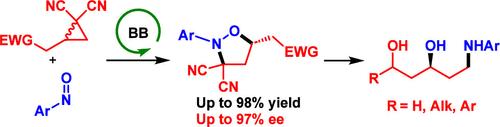
Enantioselective transformations of donor–acceptor cyclopropanes have opened a new chemical space for the construction of enantioenriched molecules. This work presents the first catalytic enantioselective synthesis of isoxazolidines─a privileged key-structure in organic and medicinal chemistry─through a Brønsted-base catalyzed (3 + 2) annulation of donor–acceptor cyclopropanes with nitrosoarenes. The reaction concept is general, scalable to gram-scale, and enables the reaction between cyclopropanes, substituted with ketones, aldehydes, esters, thioesters, or sulfones, and nitrosoarenes with different substitution patterns, yielding isoxazolidines in generally excellent yields (up to 98%) and enantioselectivities (up to 97% ee). For the (3 + 2) annulation of β-cyclopropyl ketones with nitrosobenzenes, a Hammett study was conducted to elucidate the role of substituents on enantioselectivity. The isoxazolidines can undergo different transformations, such as oxidative cleavage of the N-PMP-group or N–O bond cleavage by LiAlH4, where the two cyano groups are key-functionalities, as this reaction also provided the simultaneous didecyanation and reduction of the carbonyl, affording attractive 5-amino-1,3-diols, a scaffold present in drugs like atorvastatin. Finally, a mechanistic model is proposed to account for the stereochemical outcome of the (3 + 2) annulation.
br ønsted碱催化环丙烷与亚硝基芳烃(3 + 2)环反应形成异恶唑烷的对映选择性研究
环丙烷供体-受体的对映选择性转化为构建对映体富集分子开辟了新的化学空间。这项工作首次通过br ønsted碱催化(3 + 2)环丙烷与亚硝基芳烃的供体-受体环化,催化合成了异恶唑烷(有机化学和药物化学中的一种特殊关键结构)。反应概念是一般的,可扩展到克级,并且可以在被酮、醛、酯、硫酯或砜取代的环丙烷和具有不同取代模式的亚硝基芳之间进行反应,生成收率一般很高的异唑烷(高达98%)和对端选择性(高达97% ee)。对于β-环丙基酮与亚硝基苯的(3 + 2)环化反应,采用Hammett研究了取代基对对映体选择性的影响。异恶唑烷可以发生不同的转化,如n - pmp -基团的氧化裂解或N-O键被LiAlH4裂解,其中两个氰基是关键官能团,因为该反应还提供了羰基的同时二脱氰和还原,提供了有吸引力的5-氨基-1,3-二醇,这是一种支架,存在于阿托伐他汀等药物中。最后,提出了一个机制模型来解释(3 + 2)环化的立体化学结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


