Hexafluoroisopropanol Solvent Effects on Enantioselectivity of Dirhodium Tetracarboxylate-Catalyzed Cyclopropanation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In recent years, additives that modulate both reactivity and selectivity in rhodium-catalyzed reactions of aryldiazoacetates have become increasingly prominent. 1,1,1,3,3,3-Hexafluoroisopropanol (HFIP) has been shown to have a profound effect on rhodium carbene reactivity and selectivity, especially on enabling carbene cyclopropanation in the presence of various nucleophilic poisons. HFIP also has a variable influence on the enantioselectivity of the reactions catalyzed by chiral dirhodium tetracarboxylates, and this study examines the fundamental properties of the rhodium carbene/HFIP system through experimentation, density functional theory (DFT), and molecular dynamics (MD) simulations. These studies revealed that the C4-symmetric bowl-shaped catalysts, which have been previously considered to be relatively rigid, experience far greater flexibility in this hydrogen bonding media, resulting in distortion of the bowl-shaped catalysts. These studies explain why even though a majority of the catalysts have a drop in enantioselectivity in HFIP, some catalysts, such as Rh2(TCPTAD)4, lead to a switch in enantioselectivity, whereas others, such as Rh2(NTTL)4, lead to a considerably enhanced enantioselectivity.
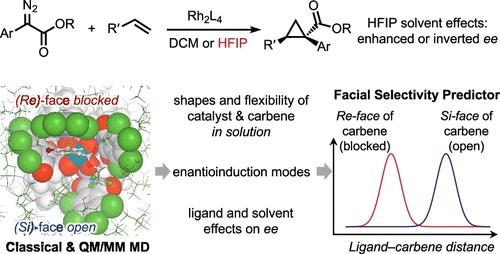
六氟异丙醇溶剂对四羧酸二钠催化环丙化反应对映选择性的影响
近年来,在芳基重氮乙酸酯的铑催化反应中,添加剂对反应活性和选择性的调节作用日益突出。1,1,1,3,3,3-六氟异丙醇(HFIP)已被证明对羰基铑的反应性和选择性有深远的影响,特别是在各种亲核毒物存在的情况下使羰基环丙烷化。HFIP对手性四羧酸迪铑催化的反应的对映选择性也有不同的影响,本研究通过实验、密度泛函理论(DFT)和分子动力学(MD)模拟来研究卡贝铑/HFIP体系的基本性质。这些研究表明,先前认为相对刚性的c4对称碗形催化剂在这种氢键介质中具有更大的灵活性,从而导致碗形催化剂的扭曲。这些研究解释了为什么即使大多数催化剂在HFIP中对映体选择性下降,一些催化剂,如Rh2(TCPTAD)4,导致对映体选择性的切换,而其他催化剂,如Rh2(NTTL)4,导致对映体选择性显著增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


