Continuous 1,4/1,6- and 1,2-addition reactions combined with the skeletal rearrangement of 5aH,13H-chromeno[2,3-b]quinolizin-13-ones with isocyanides to enable the synthesis of pyrido[2,3-b]indolizines†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00342c
引用次数: 0
Abstract
We developed a protocol for synthesizing highly functionalized pyrido[2,3-b]indolizines (PDIZs, ) from 5aH,13H-chromeno[2,3-b]quinolizin-13-ones using ((isocyanomethyl)sulfonyl)benzene derivatives. The continuous cycloaddition of [1,4], [1,6], and [1,2] bonds, along with skeletal rearrangement, was achieved by heating a mixture of substrates and in DMSO, facilitated by Cs2CO3 and catalyzed by Ag2CO3. This process led to the cleavage of three bonds (C–C, C–N, and C–O) and the formation of four bonds (C–N and three C–C) in a single step, enabling the rapid conversion of tricyclic systems from 5aH,13H-chromeno[2,3-b]quinolizin-13-ones (CMQZs) to PDIZs. The rearrangement and transformation of these scaffolds are significant for drug discovery. Additionally, the reactions can be enhanced by Ag catalysis to produce functionalized PDIZs suitable for combinatorial and parallel synthesis via one-pot reactions.
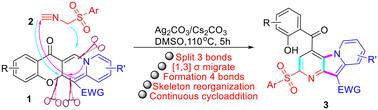
连续的1,4/1,6-和1,2-加成反应结合5aH,13H-chromeno[2,3-b]喹啉-13- 1与异氰酸酯的骨架重排,合成吡啶[2,3-b]吲哚嘧啶
我们开发了一种由5aH,13H-chromeno[2,3-b]喹啉-13- 1与(异氰基)磺酰基)苯衍生物合成高度功能化吡啶[2,3-b]吲哚嗪(PDIZs) 3的方案。[1,4]/[1,6]和[1,2]的连续环加成和骨架重排是通过在DMSO中加热底物1和2的混合物进行的,该混合物由Cs2CO3促进,Ag2CO3催化。结果表明,通过裂解3个键(C−C、C−N和C - o键),一步形成4个键(C−N和3个C−C键),制备了一系列pdiz3。该方案实现了5aH,13H-chromeno[2,3-b]喹啉-13-ones (CMQZs)三环体系到pdiz的一步转化,实现了复杂杂环的快速构建。这类支架的重排或转化对新药的发现具有重要意义。在银的催化作用下,这些反应可以产生官能化的pdiz,并通过一锅反应进行组合和平行合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


