Installation of Site-Specific Inorganic and Organic Phosphate to Peptides and Proteins via Vinylaryl Sulfonium Reagents
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Protein phosphorylation is a very important post-translational modification that regulates diverse cellular activities. In addition to classic monophosphorylation, there is also oligophosphorylation from pyrophosphorylation to polyphosphorylation. Moreover, organophosphates may modify residues such as via AMPylation and ADPylation. Although plenty of new types of protein phosphorylation have been revealed, molecular mechanisms of the biological functions are still challenging to study due to the lack of a good method to prepare proteins of interest with such site-specific PTMs. Here we report a facile method to install inorganic and organic phosphates on peptides and proteins. Vinylaryl sulfonium with an electron-withdrawing group was applied to cysteine alkylation and subsequent cyclization by γ-sulfur yielding episulfonium. This highly electrophilic intermediate was later attacked by a phosphate reagent to yield site-specifically phosphorylated cysteine peptides and proteins. As a result, this method does not require a special precursor residue on peptides/proteins or activation of phosphate reagents. In addition, this method is applicable to diverse inorganic phosphates and organophosphate. Therefore, we believe that this method will accelerate protein phosphorylation research by simple preparation of site-specific modified proteins. We also believe it offers a simple bioconjugation strategy via a phosphate linker.
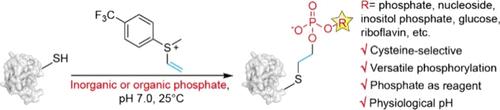
通过乙烯基芳基锍试剂将特定位点的无机和有机磷酸安装到肽和蛋白质上
蛋白质磷酸化是一种非常重要的翻译后修饰,调节着多种细胞活动。除了经典的单磷酸化外,还有从焦磷酸化到多磷酸化的寡磷酸化。此外,有机磷酸盐可以通过氨酰基化和磷酸基化等方式修饰残基。尽管已经发现了许多新的蛋白质磷酸化类型,但由于缺乏一种很好的方法来制备这种位点特异性的ptm蛋白,其生物学功能的分子机制仍然具有挑战性。在这里,我们报告了一种简便的方法来安装在肽和蛋白质上的无机和有机磷酸盐。带一个吸电子基团的乙烯基磺酸被应用于半胱氨酸烷基化和随后的γ-产硫异硫磺酸环化。这种高度亲电的中间体后来被磷酸盐试剂攻击,产生位点特异性磷酸化的半胱氨酸肽和蛋白质。因此,该方法不需要肽/蛋白质上的特殊前体残留物或磷酸试剂的激活。此外,该方法适用于多种无机磷酸盐和有机磷酸盐。因此,我们相信该方法将通过简单制备位点特异性修饰蛋白来加速蛋白质磷酸化的研究。我们也相信它提供了一个简单的生物偶联策略,通过一个磷酸盐连接。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


