Rapid Fluorochromic Sensing of Tertiary Amines and Opioids via Dual-Emissive Ground and Excited Charge-Transfer States
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The recognition and differentiation of organic amines are crucial for applications in drug analysis, food spoilage, biomedical assays, and clinical diagnostics. Existing luminescence-based recognition methods for amines predominantly rely on fluorescence quenching, limiting the scope of sensitive and selective detection. Here, we present a fluorochromic approach for rapidly distinguishing different organic amines based on their unique excited-state and ground-state interactions with a naphthalimide derivative under ultraviolet light. Our findings reveal that the photoluminescence quantum yield and emission color are significantly influenced by the substituent group and the molecular flexibility of the amine. Specifically, primary amines, together with other common lone-pair donors, such as alcohol, ether, thiol, thioether, and phosphine, did not exhibit photoluminescence changes, while secondary amines exhibited only weak emission. For tertiary amines, however, bright green photoluminescence activation was rapidly produced for molecules containing at least one methyl group; red-shifted yellow emission was observed for ones with bulkier side groups other than methyl; and for conformationally locked bicycloamines, no emission was observed. In addition, this fluorochromic process of the naphthalimide derivative not only depends on tertiary amine substituent groups but also shows distinctly different ground- and excited-state photoluminescence dynamics in time-resolved spectroscopy. Based on these differences, a qualitative method is developed for visual recognition of natural and synthetic opioids, including heroin, fentanyl, and metonitazene, which is more facile and rapid compared to current methods such as the Marquis reagent kit, and could facilitate onsite testing, real-time monitoring, and streamlined workflows in both laboratory and field settings.
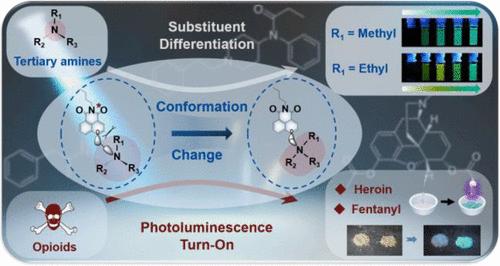
基于双发射基态和激发态的叔胺和阿片类药物的快速荧光传感
有机胺的识别和鉴别对于药物分析、食品变质、生物医学检测和临床诊断等应用至关重要。现有的基于荧光的胺类识别方法主要依赖于荧光猝灭,限制了敏感和选择性检测的范围。在这里,我们提出了一种基于其独特的激发态和基态与萘酰亚胺衍生物在紫外光下相互作用的荧光显色方法来快速区分不同的有机胺。我们的研究结果表明,取代基和胺的分子柔韧性对光致发光量子产率和发射颜色有显著影响。具体来说,伯胺与其他常见的孤对供体,如醇、乙醚、硫醇、硫醚和膦,没有表现出光致发光变化,而仲胺仅表现出弱发射。然而,对于叔胺,含有至少一个甲基的分子迅速产生明亮的绿色光致发光活化;对于除甲基外具有较大侧基的分子,观察到红移黄色发射;而对于构象锁定的双环胺,没有观察到辐射。此外,萘酰亚胺衍生物的荧光变色过程不仅依赖于叔胺取代基,而且在时间分辨光谱中表现出明显不同的基态和激发态光致发光动力学。基于这些差异,我们开发了一种定性方法,用于视觉识别天然和合成阿片类药物,包括海洛因、芬太尼和甲氧硝唑,与目前的方法(如Marquis试剂盒)相比,该方法更容易和快速,并且可以促进现场测试、实时监测,并简化实验室和现场设置的工作流程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


