Stereoselective Nickel-Catalyzed Iterative 1,2-Reduction of Trisubstituted Enones to Cycloalkanols Bearing Two Contiguous Stereocenters
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein we first report a nickel-catalyzed asymmetric iterative 1,2-reduction of trisubstituted enones to cycloalkanols with two contiguous stereocenters in high yields with excellent diastereo- and enantioselectivities (36 examples, up to 98.5:1.5 er, >20:1 dr, TON = 500). The combined experimental and computational mechanistic studies suggested energy changes during two consecutive reduction processes and provided a range of unique mechanistic rationales that have not been disclosed in nickel-catalyzed asymmetric hydrogenation-related studies.
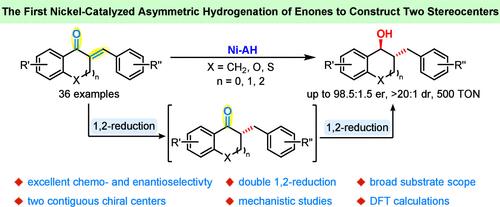
镍催化立体选择性三取代烯酮的1,2-迭代还原成具有两个连续立体中心的环烷醇
本文首次报道了镍催化的三取代烯酮的不对称迭代1,2还原成具有两个连续立体中心的环烷醇,收率高,具有优异的非立体和对映选择性(36个例子,高达98.5:1.5 er, >20:1 dr, TON = 500)。结合实验和计算机制的研究表明,在两个连续的还原过程中能量发生了变化,并提供了一系列独特的机制原理,这些原理在镍催化的不对称氢化相关研究中尚未披露。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


