Glycogen stores mediated by the p53-GYS1 feedback circuit engenders platinum resistance in ovarian clear cell carcinoma
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ovarian cancer (OC) is a highly fatal and refractory malignancy affecting women, and platinum resistance remains a major clinical dilemma. Compared with other OC subtypes, ovarian clear cell carcinoma (OCCC) frequently exhibits increased platinum refractoriness, accompanied by increased glycogen levels, which promotes clear-cell morphology, and wild-type p53. However, the roles of these factors in platinum resistance of OCCC are unclear. Here, we investigated whether glycogen promotes OCCC resistance to platinum agents and reported that GYS1, a rate-limiting enzyme in glycogen synthesis, is clinically associated with poor prognosis and chemoresistance in OCCC. Mechanistically, p53 promotes GYS1 breakdown via the upregulation of RNF144a, whereas GYS1 induces the reversal of p53 ubiquitination and degradation by competitively binding to USP14, forming a positive feedback circuit. Under platinum stress, the accumulated glycogen is mobilized by the p53/GYS1 feedback circuit, which fuels energetic NADPH production, resulting in resistance to disulfidptosis and increased platinum resistance in OCCC. Collectively, our findings identify glycogen as a contributor to OCCC platinum resistance and elucidate the underlying mechanisms, highlighting a crucial p53/GYS1 positive feedback loop.
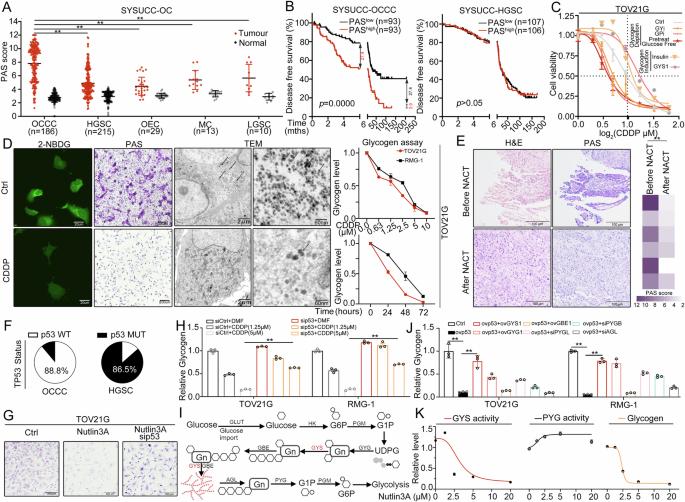

p53-GYS1反馈回路介导的糖原储存在卵巢透明细胞癌中产生铂耐药
卵巢癌(OC)是一种高致死率和难治性的女性恶性肿瘤,铂耐药一直是临床的主要难题。与其他OC亚型相比,卵巢透明细胞癌(OCCC)经常表现出铂的难治性增加,并伴有糖原水平升高,从而促进透明细胞形态和野生型p53。然而,这些因素在OCCC铂电阻中的作用尚不清楚。在这里,我们研究了糖原是否促进了OCCC对铂类药物的耐药性,并报道了GYS1(糖原合成中的限速酶)在临床上与OCCC的不良预后和化疗耐药相关。机制上,p53通过上调RNF144a促进GYS1的分解,而GYS1通过竞争性结合USP14诱导p53泛素化和降解的逆转,形成一个正反馈回路。在铂胁迫下,积累的糖原被p53/GYS1反馈回路动员起来,为NADPH的产生提供能量,导致OCCC对二硫磷酸酯的抗性和对铂的抗性增加。总的来说,我们的研究结果确定糖原是OCCC铂耐药的一个因素,并阐明了潜在的机制,强调了关键的p53/GYS1正反馈回路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


