Proteomimetic Strategy for the Modulation of Intrinsically Disordered Protein MYC
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
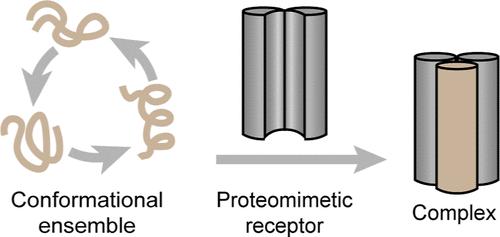
The difficulty in developing specific ligands for protein receptors is directly correlated to the presence of unique binding sites on the protein surface. Conformationally dynamic proteins increase the level of difficulty in ligand design, and the challenge is further exacerbated for proteins that are intrinsically disordered. Intrinsically disordered proteins (or IDPs) do not adopt a fixed three-dimensional shape until they bind their target; an absence of organized binding sites underscores the difficulty in developing synthetic ligands for these proteins. We hypothesized that one avenue for the development of binders for a disordered region would be to trap one of its thermodynamically accessible conformations in a receptor. Here, we show the application of this approach to MYC, which represents a critical therapeutic target but has not yielded small-molecule inhibitors due to its conformationally dynamic nature. MYC adopts a helical configuration when it binds to its cellular partner MAX. We rationally designed a proteomimetic scaffold to trap this conformation. We show that MYC can be directly engaged in both biochemical and cellular assays. Overall, this work demonstrates a general method to capture and trap intrinsically disordered proteins with a propensity to adopt α-helical conformations.
调控本质紊乱蛋白 MYC 的拟蛋白策略
为蛋白质受体开发特异性配体的困难与蛋白质表面存在独特的结合位点直接相关。构象动态蛋白质增加了配体设计的难度,而对于本质上无序的蛋白质,这一挑战进一步加剧。内在无序的蛋白质(或IDPs)在与靶标结合之前不会采用固定的三维形状;缺乏有组织的结合位点强调了为这些蛋白质开发合成配体的困难。我们假设,为无序区域开发粘合剂的一种途径是在受体中捕获其热力学可接近的构象之一。在这里,我们展示了这种方法在MYC中的应用,MYC是一个关键的治疗靶点,但由于其构象动力学性质,尚未产生小分子抑制剂。当MYC与细胞伴侣MAX结合时,它采用螺旋结构。我们合理地设计了一种模拟蛋白质的支架来捕获这种构象。我们发现MYC可以直接参与生化和细胞分析。总的来说,这项工作证明了一种捕获和捕获具有采用α-螺旋构象倾向的内在无序蛋白质的一般方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


