The intrinsic expression of NLRP3 in Th17 cells promotes their protumor activity and conversion into Tregs
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Th17 cells can perform either regulatory or inflammatory functions depending on the cytokine microenvironment. These plastic cells can transdifferentiate into Tregs during inflammation resolution, in allogenic heart transplantation models, or in cancer through mechanisms that remain poorly understood. Here, we demonstrated that NLRP3 expression in Th17 cells is essential for maintaining their immunosuppressive functions through an inflammasome-independent mechanism. In the absence of NLRP3, Th17 cells produce more inflammatory cytokines (IFNγ, Granzyme B, TNFα) and exhibit reduced immunosuppressive activity toward CD8+ cells. Moreover, the capacity of NLRP3-deficient Th17 cells to transdifferentiate into Treg-like cells is lost. Mechanistically, NLRP3 in Th17 cells interacts with the TGF-β receptor, enabling SMAD3 phosphorylation and thereby facilitating the acquisition of immunosuppressive functions. Consequently, the absence of NLRP3 expression in Th17 cells from tumor-bearing mice enhances CD8 + T-cell effectiveness, ultimately inhibiting tumor growth.
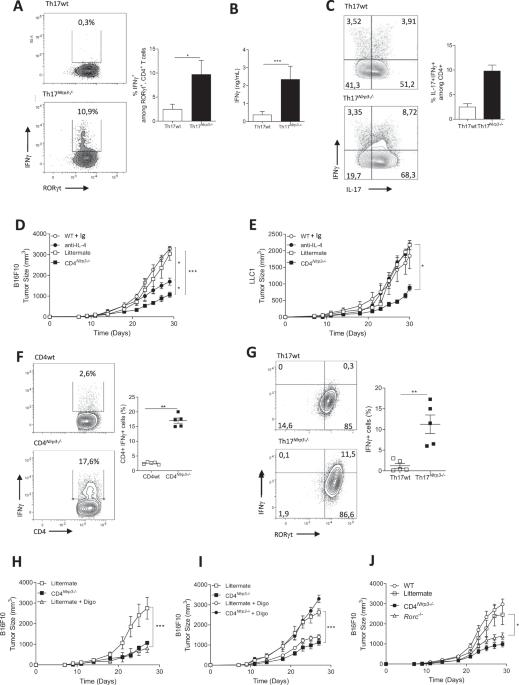
NLRP3在Th17细胞中的内在表达促进了它们的肿瘤活性和向Tregs的转化。
Th17细胞可以根据细胞因子微环境发挥调节或炎症功能。这些可塑性细胞可以在炎症消退过程中,在同种异体心脏移植模型中,或在癌症中通过尚不清楚的机制转化为treg。在这里,我们证明了NLRP3在Th17细胞中的表达是通过炎性小体独立机制维持其免疫抑制功能所必需的。在NLRP3缺失的情况下,Th17细胞产生更多的炎症因子(IFNγ、颗粒酶B、TNFα),对CD8+细胞的免疫抑制活性降低。此外,nlrp3缺失的Th17细胞转分化为treg样细胞的能力丧失。在机制上,Th17细胞中的NLRP3与TGF-β受体相互作用,使SMAD3磷酸化,从而促进免疫抑制功能的获得。因此,荷瘤小鼠Th17细胞中NLRP3表达缺失可增强CD8 + t细胞的有效性,最终抑制肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


