Photocatalytic three-component 1,2-boroarylation and carbopyridylation of alkene†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-15
DOI:10.1039/d5qo00380f
引用次数: 0
Abstract
In the present work, we developed a photocatalytic three-component 1,2-boroarylation of alkenes using readily available alkenes, bis(pinacolato)diboron, and (hetero)aryl nitriles in the presence of base and photocatalyst—to afford a diverse array of boryl-enriched 1,1-diarylalkane compounds. Alkylboronic pinacol esters were also found to be suitable substrates for the reaction, giving the corresponding carbopyridylation products. Control experiments and DFT calculations supported our proposed photoinduced generation of a boryl unit and aryl nitrile radical anion, which sequentially coupled to alkene. This protocol exhibited mild reaction conditions, good functional group tolerance and a one-pot procedure, serving as a complementary approach to transition-metal-catalyzed coupling reaction.
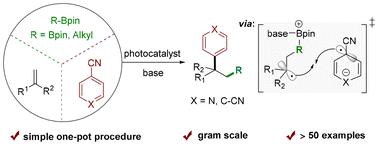
光催化烯烃的三组分1,2 -硼芳基化和碳吡啶化
在此,我们提出了在碱和光催化剂的存在下,用易得的烯烃、双(pinacolato)二硼和(杂)芳基腈进行三组分1,2-硼芳基化的光催化,得到了多种富含硼的1,1-二芳烷化合物。烷基硼蒎醇酯也是生成相应的碳吡啶化产物的合适底物。对照实验和DFT计算支持我们提出的光诱导一代的硼基单元和芳基腈自由基阴离子,顺序偶联到烯烃。该方案具有反应条件温和、功能耐受性好、一锅法等特点,是过渡金属催化偶联反应的补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


