Molecular Compasses for Modulating Electronic Communication in Pillar[5]quinone
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
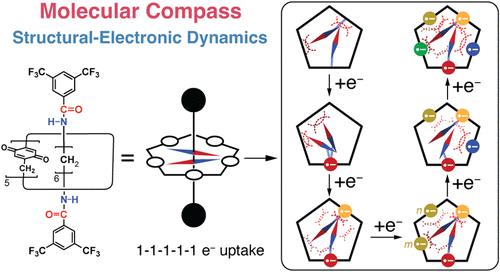
Just as a pointer, which moves freely and points to the magnetic North in a compass, affords us with a device for tracking direction on a global scale, a dipole moment in a molecule is capable of aligning itself in a compass-like manner in response to an electric field at the molecular level. Here, we demonstrate that dipole moment pointers, based on amide and ester groups in the dumbbell components of [2]rotaxanes, are susceptible to changes in pole–dipole and dipole–dipole interactions within a redox-active pillar[5]quinone ring component when subjected to redox control. Distinct from free pillar[5]quinone, these molecular compasses exhibit a 1–1–1–1–1 electron-uptake pattern during the first-electron transfers. Density functional theory (DFT) calculations reveal that, upon the reduction of quinoid units in the amide-based molecular compass, the positive end of the dipole moment pointer in the dumbbell component becomes oriented toward the reduced anionic quinone in the ring component, courtesy of hydrogen bonding. The negative end of the dipole moment pointer in the dumbbell component lowers the reduction potentials of the other four quinoid units as a result of electrostatic repulsions, which explain its 1–1–1–1–1 electron-uptake pattern. Our findings highlight how electronic communication between the dipole moment pointers and the quinoid units in the ring component enables the [2]rotaxane to act as a molecular compass, precisely reorienting its dipole moments in response to redox changes.
用分子罗盘调制[5]醌柱中的电子通信
就像指南针上自由移动并指向磁北的指针为我们提供了一种在全球范围内跟踪方向的装置一样,分子中的偶极矩能够以类似指南针的方式对分子水平上的电场作出反应。在这里,我们证明了偶极矩指针,基于[2]轮烷哑铃组分中的酰胺和酯基团,在氧化还原活性柱[5]醌环组分中,当受到氧化还原控制时,容易受到极-偶极子和偶极子-偶极子相互作用的变化。与自由柱[5]醌不同,这些分子罗盘在第一电子转移过程中呈现出1-1-1-1-1的电子摄取模式。密度泛函理论(DFT)计算表明,在酰胺基分子罗盘中,由于氢键作用,哑铃组分偶极矩指针的正端指向环组分中被还原的阴离子醌。哑铃组分偶极矩指针的负端由于静电斥力降低了其他四个类醌单元的还原电位,这解释了其1-1-1 - 1的电子吸收模式。我们的发现强调了环组分中偶极矩指针和类醌单位之间的电子通信如何使[2]轮烷作为分子指南针,精确地重新定向偶极矩以响应氧化还原变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


