Is dystrophin immunogenicity a barrier to advancing gene therapy for Duchenne muscular dystrophy?
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Duchenne muscular dystrophy (DMD) is a neuromuscular disorder that leads to severe disability and premature death in young men. As DMD is caused by the absence of dystrophin, therapeutic development has focused on strategies to restore dystrophin expression. These include readthrough of premature stop codons, exon skipping to restore the reading frame, and gene therapy. The first two methods are mutation-specific, benefiting only subsets of patients, whereas gene therapy could treat all individuals with DMD. Immunogenicity of dystrophin may challenge these efforts. The immune system can recognize dystrophin as a neo-antigen, just as it can recognize newly arising antigens present on mutated cells. An in-depth evaluation of anti-dystrophin immune response as a factor affecting the treatment effectiveness is needed. Key questions include the underlying mechanisms of immunity induction by antigenic epitopes of the re-expressed dystrophin, the impact of such responses on the therapeutic efficacy, and the role of patient-specific risk factors, such as preimmunization due to revertant fibres, chronic muscle inflammation, pre-existing T lymphocytes reactive to dystrophin, which avoided deletion in dystrophic thymus, or antigen cross-reactivity. Patients’ immune status assessment before treatment may help mitigating anti-dystrophin responses. Exploring potential therapeutic strategies to enhance treatment outcomes is also essential: Since DMD can be diagnosed at birth, early dystrophin re-expression could prevent damage and also potentially induce neonatal tolerance. In older patients, carefully managed immunosuppression and tolerogenic protocols could pave the way for more successful dystrophin replacement therapies.
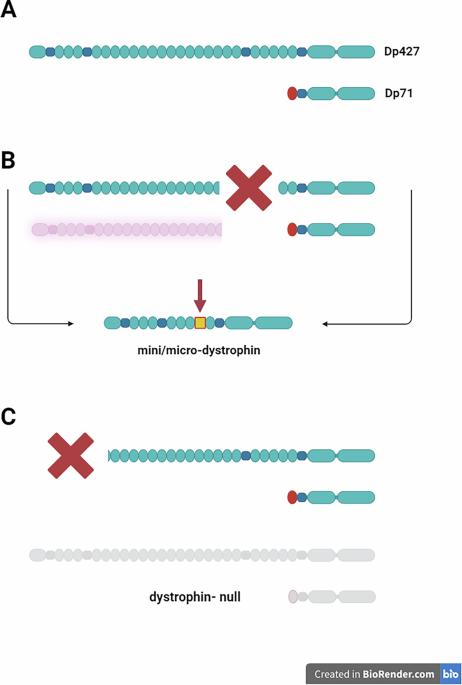
肌营养不良蛋白免疫原性是推进杜氏肌营养不良基因治疗的障碍吗?
杜氏肌营养不良症(DMD)是一种导致年轻男性严重残疾和过早死亡的神经肌肉疾病。由于DMD是由肌营养不良蛋白缺乏引起的,治疗发展的重点是恢复肌营养不良蛋白表达的策略。这些方法包括读取过早终止密码子,外显子跳跃以恢复阅读框,以及基因治疗。前两种方法是突变特异性的,只对一部分患者有益,而基因疗法可以治疗所有的DMD患者。肌营养不良蛋白的免疫原性可能会挑战这些努力。免疫系统可以识别肌营养不良蛋白作为一种新抗原,就像它可以识别突变细胞上新产生的抗原一样。需要深入评估抗肌营养不良蛋白免疫反应作为影响治疗效果的因素。关键问题包括再表达的肌营养不良蛋白抗原表位诱导免疫的潜在机制,这种反应对治疗效果的影响,以及患者特异性风险因素的作用,如由于纤维逆转而预先免疫,慢性肌肉炎症,预先存在的T淋巴细胞对肌营养不良蛋白反应,避免在营养不良胸腺中缺失,或抗原交叉反应。患者治疗前的免疫状态评估可能有助于减轻抗肌营养不良反应。探索潜在的治疗策略以提高治疗效果也很重要:由于DMD可以在出生时诊断出来,因此早期肌营养不良蛋白的再表达可以预防损伤,也可能诱导新生儿耐受。在老年患者中,精心管理的免疫抑制和耐受性方案可以为更成功的肌营养不良蛋白替代疗法铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


