Temporal interactions between neural proxies for memory recall, negative affect, and emotion regulation in major depression
IF 10.1
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
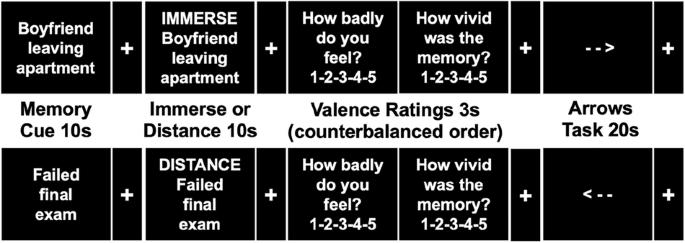
Dysfunction in emotion regulation (ER) and autobiographical memory are components of major depressive disorder (MDD). However, little is known about how they mechanistically interact with mood disturbances in real time. Using machine learning-based neural signatures, we can quantify negative affect (NA), ER, and memory continuously to evaluate how these processes dynamically interact in MDD. Unmedicated individuals with MDD (N = 45) and healthy volunteers (HV; N = 38) completed a negative autobiographical memory functional magnetic resonance imaging task wherein they recalled, distanced from (an ER strategy), and immersed into memories. We used a negative affect signature (PINES) and an emotion regulation signature (ERS) to quantify moment-to-moment NA and ER. We then examined whether memory engagement, indexed by hippocampal activity, predicted subsequent change in PINES and ERS over time. During memory recall and immersion, greater hippocampal activity predicted increased PINES across groups. During distancing, greater hippocampal activity in HVs predicted increased ERS but not PINES. In MDD, greater hippocampal activity predicted increased PINES but not ERS. Findings suggest abnormalities in the real-time relationship between memory, NA, and ER in MDD. During distancing, as predicted, HVs showed an attenuation of the linkage between memory engagement and NA, and they had subsequent increases in ER following memory reactivation. In contrast, MDD was characterized by continued linkage between memory engagement and NA, without subsequent increases in ER. Deficits in engagement of ER and ineffective modulation of NA following negative memory recall may contribute to the mood disturbances in MDD and are potential targets for clinical intervention.

重度抑郁症患者记忆回忆、负面情绪和情绪调节的神经代理的时间交互作用
情绪调节功能障碍(ER)和自传体记忆功能障碍是重度抑郁症(MDD)的组成部分。然而,人们对它们如何与情绪障碍实时相互作用知之甚少。利用基于机器学习的神经特征,我们可以连续量化负面影响(NA)、ER和记忆,以评估这些过程如何在MDD中动态相互作用。未服药的重度抑郁症患者(N = 45)和健康志愿者(HV;N = 38)完成了一项负性自传体记忆功能磁共振成像任务,其中他们回忆,远离(ER策略),沉浸在记忆中。我们使用负面情绪特征(PINES)和情绪调节特征(ERS)来量化瞬时NA和ER。然后,我们检查了海马活动索引的记忆参与是否预测了随后PINES和ERS随时间的变化。在记忆回忆和沉浸过程中,海马活动的增加预示着各组间PINES的增加。在距离过程中,hv的海马活动增加预示着ERS增加,而不是PINES增加。在重度抑郁症中,海马活动的增加预示着PINES的增加,而不是ERS的增加。研究结果表明,MDD患者的记忆、NA和ER之间的实时关系存在异常。在保持距离期间,正如预测的那样,HVs表现出记忆参与和NA之间联系的衰减,并且在记忆再激活后,他们随后的ER增加。相比之下,MDD的特点是记忆参与和NA之间的持续联系,没有随后的ER增加。负性记忆回忆后内质网参与的缺陷和NA的无效调节可能导致重度抑郁症的情绪障碍,是临床干预的潜在目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


