ALKBH5-mediated m6A regulates the alternative splicing events of SRSF10 in ovarian cancer
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
N6-methyladenosine (m6A) methylation was found to be involved in the tumorigenesis and development of ovarian cancer. Until now, it is not clear to identify the mechanism by m6A demethylase ALKBH5 affects RNA splicing in ovarian cancer. In this study, we examined ALKBH5 protein expression and m6A levels by immunohistochemistry and analyzed their correlation with clinical features and prognosis in patients with ovarian cancer. We identified the elevated expression of ALKBH5 and a general reduction in the level of m6A in ovarian cancer patients. In the ovarian cancer cell line A2780, ALKBH5 depletion was found using the siRNA strategy or the CRISPR/Cas9 knockout (KO) method, which significantly reduced the level of m6A and inhibited cell viability, proliferation, and migration. The MeRIP-seq and RNA-seq showed that ALKBH5-regulated m6A RNA modification mainly affects RNA splicing function in ovarian cancer cells. SRSF10 is a key target gene involved in alternative splicing regulation through ALKBH5-m6A. ALKBH5 knockdown resulted in increased retention of SRSF10 exon 5 and decreased expression of transcript SRSF10-211. In conclusion, the alternative splicing regulation effect by ALKBH5-mediated m6A suggests a novel promising approach for m6A modification in OC and provides novel insights into the mechanisms involved in ovarian cancer therapy.
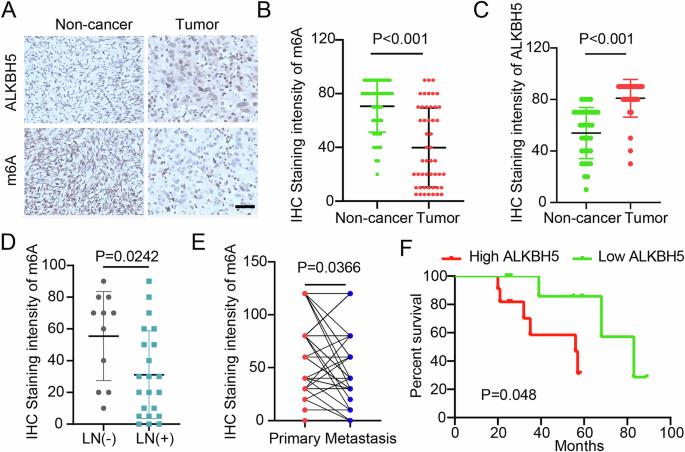
alkbh5介导的m6A调控SRSF10在卵巢癌中的选择性剪接事件。
发现n6 -甲基腺苷(m6A)甲基化参与卵巢癌的发生和发展。迄今为止,尚不清楚m6A去甲基化酶ALKBH5在卵巢癌中影响RNA剪接的机制。本研究通过免疫组化检测ALKBH5蛋白表达及m6A水平,分析其与卵巢癌患者临床特征及预后的相关性。我们发现在卵巢癌患者中ALKBH5的表达升高,而m6A的水平普遍降低。在卵巢癌细胞系A2780中,使用siRNA策略或CRISPR/Cas9敲除(KO)方法发现ALKBH5缺失,显著降低m6A水平,抑制细胞活力、增殖和迁移。MeRIP-seq和RNA-seq结果显示,alkbh5调控的m6A RNA修饰主要影响卵巢癌细胞的RNA剪接功能。SRSF10是通过ALKBH5-m6A参与选择性剪接调控的关键靶基因。ALKBH5敲低导致SRSF10外显子5的保留增加,转录物SRSF10-211的表达降低。总之,alkbh5介导的m6A的选择性剪接调节作用为卵巢癌中m6A修饰提供了一种新的有希望的方法,并为卵巢癌治疗的机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


