Synthesis of All Ring Sizes of Medium-Sized Heterocycles Bridged Biaryls via VQM-Enabled Diversity-Oriented Synthetic Strategy
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, for the first time, the controllable, accurate, and diverse synthesis of all ring sizes of medium-sized (8- to 11-membered) indole-derived bridged biaryls has been realized by using ingeniously designed o-alkynylnaphthols that feature cyclic amines with adjustable ring sizes. The transformation may proceed through a DBN-mediated in-situ generation of vinylidene ortho-quinone methides/indole-ring formation/ring expansion cascade sequence, which is characterized by acceptable to excellent yields and good functional group tolerance.
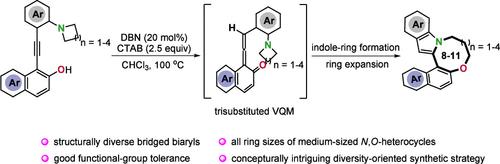
基于vqm的多样性导向合成策略合成所有环尺寸的中型杂环桥接双芳
本研究首次利用巧妙设计的环胺可调节环尺寸的邻炔基萘酚,实现了中等大小(8- 11元)吲哚衍生桥联芳基所有环尺寸的可控、准确和多样化合成。该转化过程可能通过dbn介导的原位生成偏二邻醌类化合物/吲哚环形成/扩环级联序列进行,具有可接受的优异产率和良好的官能团耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


