NHC and photoredox co-catalyzed 1,4-alkylcarbonylation of 1,3-enynes†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d4qo02311k
引用次数: 0
Abstract
A strategy to obtain 1,2-alkenyl ketone products by radical relay coupling is proposed. We report that the ketyl radical produced by the single electron reduction of acyl azolium mediated by NHCs is a good coupling partner of alkenyl radicals, and the imidazole ions generated in this process directly activate alkylboronic acids to form alkyl radicals. This transformation mode is carried out under mild conditions and shows an excellent range of substrate applications.
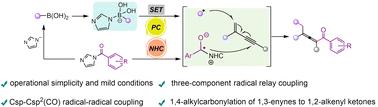
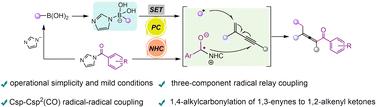
NHC和光氧化还原共催化1,3-炔的1,4-烷基羰基化反应
提出了一种采用自由基接力偶联法制备1,2-烯基酮产品的方法。我们报道了NHCs介导的酰基唑单电子还原产生的烷基自由基是烯基自由基的良好偶联伙伴,该过程中产生的咪唑离子直接激活烷基硼酸形成烷基自由基。这种转换模式在温和的条件下进行,并显示出良好的衬底应用范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


