Few-Atom Copper Cluster Facilitates H2O2 Activation to Promote Selective Oxidation of Benzene to Phenol
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The catalytic oxidation of benzene faces challenges in achieving high activity and selectivity. While single-atom catalysts present intriguing potential for this transformation, their practical implementation is hindered by intrinsic limitations in the mass-specific activity. In this context, few-atom cluster catalysts have emerged as an alternative, leveraging well-defined metal ensemble effects that enable precisely tailored active sites and enhanced interatomic synergies. Herein, we introduce an atomic cluster supported on a graphitic carbonitride (CN) catalyst (Cu3/CN), exhibiting excellent catalytic performance for selective oxidation of benzene to phenol, with superior turnover frequency (TOF) to that of single-atom Cu1/CN (719 h–1 vs 280 h–1) and suppressing phenol selectivity to that of nanoparticle CuNP/CN (95.3% vs 77.2%). Multimodal mechanistic investigations unambiguously identify the critical role of the adsorbed O* on the Cu site (Cu═O*) for C–H-oxidation, verified by both in situ spectroscopic monitoring and ex situ surface analysis. Complementary density functional theory calculations validate Cu atomic cluster (Cu3) site features a higher d-band center and larger charge transfer with the H2O2 molecule than that of the isolated Cu1 site. The sufficient charge transfer stretches the O–O bond in H2O2 to facilitate the formation of the Cu═O* species. Furthermore, the resulting Cu═O* in the Cu3 site demonstrates a significant hybridization of the O 2p orbitals and Cu 3d orbitals at the Fermi level, which endows it with high activity for benzene activation. The appropriate ensemble effect of the unique Cu3 architecture is the key to its higher catalytic performances. This work establishes a structure–performance correlation that highlights the critical role of atomic cluster architecture in optimizing catalytic functionality.
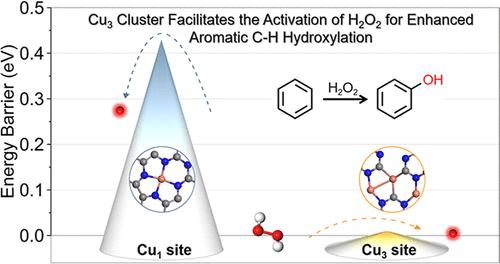
小原子铜簇有助于 H2O2 活化,促进苯对苯酚的选择性氧化
苯的催化氧化在实现高活性和选择性方面面临挑战。虽然单原子催化剂在这一转变方面表现出令人着迷的潜力,但它们的实际应用受到质量比活性的内在限制。在这种情况下,少原子团簇催化剂作为一种替代方案出现,利用定义明确的金属系综效应,实现精确定制的活性位点和增强的原子间协同作用。在此,我们引入了一种基于石墨碳氮化物(CN)催化剂(Cu3/CN)的原子团簇,该原子团簇对苯选择性氧化为苯酚表现出优异的催化性能,其转换频率(TOF)优于单原子Cu1/CN (719 h-1 vs 280 h-1),并且对苯酚的选择性抑制优于纳米颗粒cup /CN (95.3% vs 77.2%)。多模态机理研究明确地确定了吸附在Cu位点(Cu = O*)上的O*在c - h氧化中的关键作用,并通过原位光谱监测和非原位表面分析进行了验证。互补密度泛函理论计算验证了Cu原子簇(Cu3)位点比孤立的Cu1位点具有更高的d波段中心和更大的与H2O2分子的电荷转移。充分的电荷转移可拉伸H2O2中的O - O键,从而促进Cu = O*物质的形成。此外,Cu3位点上的Cu = O*在费米能级上显示了O 2p轨道和Cu 3d轨道的显著杂化,这使它具有较高的苯活化活性。独特的Cu3结构的适当的系综效应是其提高催化性能的关键。这项工作建立了结构-性能相关性,强调了原子簇结构在优化催化功能中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


