Dimethoxytetramethyldisilane: overcoming the limitations of palladium-catalyzed C–H silacyclization of 2-iodobiphenyls†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00249d
引用次数: 0
Abstract
Herein, a readily accessible silicon reagent (dimethoxytetramethyldisilane) has been developed for time-controlled palladium-catalyzed C–H silacyclization of 2-iodobiphenyls. This protocol enables divergent synthesis of dibenzooxadisilepines and dibenzosiloles in moderate to excellent yields by a process involving palladium-catalyzed disilylation, hydrolysis, condensation, and ring contraction. Notably, this reaction is compatible with a variety of substrates with electron-withdrawing groups, which overcomes the limitations of previous reports.
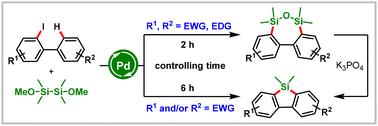
二甲氧基四甲基二硅烷:克服钯催化的2-碘联苯C-H硅环化的局限性
本文开发了一种易于获得的硅试剂(二甲氧基四甲基二硅烷),用于时间控制钯催化的2-碘联苯的C−H硅环化。该方案通过钯催化的二苯乙烯化、水解、缩合和环收缩过程,以中等至优异的收率合成二苯并二苯甲二苯甲嘧啶和二苯甲硅酮。值得注意的是,该反应与多种具有吸电子基团的底物相容,克服了以往报道的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


