A Fresh Twist on the Phospha-(Aza)-Wittig Reaction
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reactivity of an unsupported phosphinidene oxide, BnArNP═O (Bn = benzyl; Ar = bulky aryl group), as the electrophilic partner in Wittig reactions with ylides is described. Reactions with methylene-triphenylphosphorane (H2C═PPh3) and ethylidene-triphenyl-phosphorane (HMeC═PPh3), proceed as expected, giving rise to the phosphaalkene metathesis products and triphenylphosphine oxide. This reaction can be extended to other ylides such as N-(triphenylphosphoranylidene)methanamine (MeN═PPh3), to afford an aminoiminophosphane BnArNP═NMe. In these reactions the phosphinidene oxide plays the role of an electrophile, which would typically be the remit of an organic carbonyl in classical Wittig reactions. Further mechanistic insight into such transformations can be gained by altering the nature of the phosphorus-ylide. Upon reacting BnArNP═O with H2C═PMe3 (which possesses a smaller, more Lewis basic, phosphine) an alternative product is formed. This transformation supports the formation of a betaine intermediate that subsequently undergoes hydrogen-migration to afford an oxidized phosphorus(V) compound related to phosphorus acid.
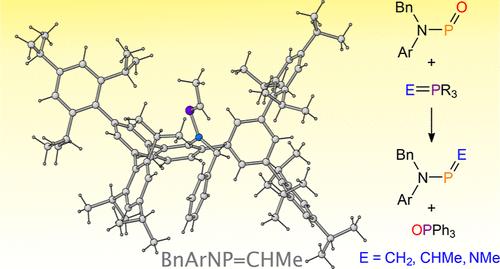
磷酸-(Aza)-维蒂格反应的新进展
本文介绍了一种无支撑的亚膦氧化物 BnArNP═O(Bn = 苄基;Ar = 大芳基)作为亲电伴侣在与酰化物的维蒂希反应中的反应活性。与亚甲基三苯基膦(H2C═PPh3)和亚乙基三苯基膦(HMeC═PPh3)的反应按预期进行,生成膦烷烃偏聚产物和三苯基氧化膦。该反应可扩展到其他酰化物,如 N-(三苯基膦亚基)甲胺(MeN═PPh3),从而得到氨基亚氨基膦 BnArNP═NMe。在这些反应中,氧化亚膦扮演了亲电体的角色,而这通常是经典维蒂希反应中有机羰基的职责。通过改变磷酰的性质,可以从机理上进一步了解此类转化。当 BnArNP═O 与 H2C═PMe3(H2C═PMe3 具有更小的路易斯碱性膦)反应时,会形成另一种产物。这种转化支持甜菜碱中间体的形成,随后甜菜碱中间体发生氢迁移,生成一种与磷酸有关的氧化磷(V)化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


