Construction of chiral γ-lactam scaffolds via asymmetric cascade [3 + 2] annulation of N-alkoxyacrylamides catalyzed by a chiral-at-metal rhodium complex†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00276a
引用次数: 0
Abstract
An efficient asymmetric cascade [3 + 2] annulation of N-alkoxyacrylamides with α,β-unsaturated 2-acyl imidazoles catalyzed by a chiral-at-metal rhodium complex has been developed. Enantioenriched γ-lactam derivatives bearing three contiguous tertiary stereocenters were obtained in generally high yields (up to 95%) and good stereoselectivities (up to >20 : 1 dr, 97% ee). Remarkably, as little as 0.2 mol% of the chiral Rh(iii) complex enables a gram-scale reaction with good yield and enantioselectivity. This reaction represents the first catalytic asymmetric [3 + 2] annulation of N-alkoxyacrylamides using a chiral Lewis acid as the catalyst, which will expand the scope of catalytic asymmetric reactions of N-alkoxyacrylamide derivatives.
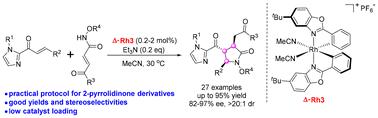
手性金属铑配合物催化n -烷氧基丙烯酰胺不对称级联[3+2]环构建手性γ-内酰胺支架
研究了手性金属铑配合物催化n -烷氧基丙烯酰胺与α,β-不饱和2-酰基咪唑的高效不对称级联[3+2]环反应。对映体富集的γ-内酰胺衍生物具有三个连续的三级立体中心,收率一般较高(高达95%),立体选择性良好(高达>;20:1 dr, 97% ee)。该反应首次实现了手性Lewis酸催化n -烷氧基丙烯酰胺的不对称[3+2]环化,将丰富n -烷氧基丙烯酰胺衍生物催化不对称反应的研究领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


