Fe-Catalyzed B–H and N–H insertion reactions of iodonium ylides†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d4qo01916d
引用次数: 0
Abstract
Herein, B–H and N–H insertion reactions of iodonium ylides in the presence of an iron catalyst complexed with imidazo[1,5-a]pyridine are developed, achieving the borylation and amination of 1,3-dicarbonyl derivatives under mild reaction conditions. Mechanistic investigations show that an iron carbene is involved in this process. Besides, this reaction features high efficiency, a broad substrate scope and good functional group tolerance, and propionate ester scaffolds can be introduced into various drug molecules via this protocol.
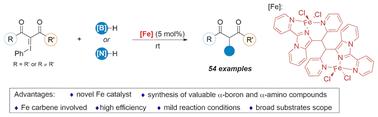
铁催化碘化物的B-H和N-H插入反应
烯烃的双官能化作为一种有效的合成策略已经引起了有机合成化学家的极大关注,从而使复杂的分子支架能够在一个简单的步骤中从容易获得的原料化学品中组装出来。本文研究了镍催化bn -杂环烯烃的还原双官能化反应。利用选择的双官能化反应的特性合成了多种bn -杂环化合物。从而通过进一步的转化和应用,能够合成范围更广的bn -杂环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


