Catalytic Schmittel-Type [2+2] Cycloaddition of γ-Alkynyl Diazoacetates with Terminal Alkynes for Accessing Cyclobuta[a]indenes
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Instead of the conventional [4+2] cycloaddition, a regioselective Schmittel-type [2+2] cycloaddition of yne–allene esters, generated in situ from copper-catalyzed dediazotized coupling of γ-alkynyl diazoacetates with terminal alkynes, is reported, enabling a bicyclization process to produce a diverse array of C1-arylated cyclobuta[a]indenes in moderate to good yields. The protocol features wide functional group compatibility, mild reaction conditions, and experimental simplicity, holding significant potential for building new tricyclic cyclobutenes.
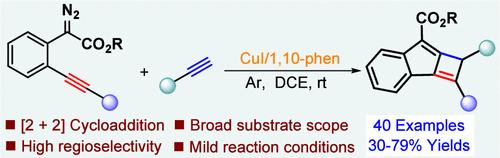
末端炔烃催化γ-炔基重氮乙酸酯的schmittel型[2+2]环加成反应制备环丁酸[a]化合物
取代传统的[4+2]环加成,一种区域选择性的schmittel型[2+2]炔烯酯环加成,由铜催化γ-炔基重氮乙酸酯与末端炔的去重氮偶联原位生成,使双环化过程能够以中等到良好的产率生产各种c1 -芳基化环丁酸[a]。该方案具有官能团相容性广、反应条件温和、实验简单等特点,具有构建新的三环丁烯的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


