Metallaphotoredox-enabled enantioselective aryldifluoromethyl-alkynylation of alkenes via C(sp3)–F bond activation†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00370a
引用次数: 0
Abstract
Due to the prevalence of trifluoromethylarene (ArCF3) units in drug-related molecules, direct late-stage functionalization of C(sp3)–F bonds in ArCF3 offers an appealing strategy for rapid derivatization of ArCF3-containing drugs, and holds significant promise in drug discovery and modification. Following the success of two-component reactions for C(sp3)–F bond functionalization in ArCF3, preliminary studies have also been conducted on non-asymmetric three-component reactions. However, enantioselective three-component reactions via C(sp3)–F bond activation in ArCF3 have not yet been developed. Herein, we report the first enantioselective three-component aryldifluoromethyl-alkynylation of alkenes through C(sp3)–F bond cleavage via dual photoredox/copper catalysis. This protocol is compatible with a wide array of trifluoromethylarenes bearing diverse substituents, various terminal alkynes and alkenes, enabling straightforward access to structurally diverse ArCF2-containing propargylic compounds in good yields with excellent enantioselectivities under mild conditions. Furthermore, the utility of this protocol was showcased through its application in the late-stage functionalization of a few drugs and bioactive molecular derivatives.
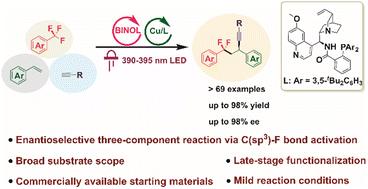
通过C(sp3)-F键激活的金属光氧化还原使芳基二氟甲基烷基化烯烃的对映选择性
由于三氟甲基芳烃(ArCF3)单元在药物相关分子中的普遍存在,直接后期功能化ArCF3中的C(sp3)-F键为含ArCF3的药物的快速衍生化提供了一种有吸引力的策略,并且在药物发现和修饰方面具有重要的前景。继ArCF3中C(sp3)-F键功能化的双组份反应成功后,非对称三组份反应也进行了初步研究。然而,该领域的对映选择性三组分反应尚未发展起来。在这里,我们报道了第一个通过双光氧化还原/铜催化C(sp3)-F键裂解烯烃的对映选择性三组分芳基二氟甲基烷基化反应。该工艺与多种含不同取代基的三氟甲基芳烃、各种末端炔和烯烃兼容,可以在温和条件下以高产量和优异的对映选择性直接获得结构多样的含arcf2丙炔化合物。此外,通过在一些药物和生物活性分子衍生物的后期功能化应用,该协议的效用得到了展示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


