Ni-catalyzed regio- and diastereoselective syn-alkynylamination of unactivated alkenes using alkylamine sources†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00194c
引用次数: 0
Abstract
The three-component alkynylamination of alkenes is a prevalent and powerful platform for rapid access to homopropargyl amines. However, the use of alkylamines to achieve this transformation remains a significant challenge. Here, we report a nickel-catalyzed intermolecular selective alkynylamination of unactivated alkenes to access the corresponding homopropargyl amines as single diastereomers. This protocol offers a range of alkylamine sources, including secondary and primary alkyl amines, as well as even the less sterically hindered primary amines, and demonstrates high functional group compatibility with alkynylsilanes. Additionally, the reaction can be applied to late-stage modifications of natural products and drugs, resulting in good yields and excellent diastereoselectivities.
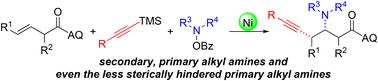
镍催化非活化烯烃与烷基胺源的区域和非对映选择性同构烷基胺层合反应
烯烃的三组分烷基层化为快速获得同丙基胺提供了一个普遍而强大的平台。然而,使用烷基胺来实现这种转化仍然是一个重大的挑战。在这里,我们报道了镍催化的非活化烯烃的分子间选择性烷基层化,以获得相应的同丙基胺作为单一的非对映体。该方案提供了一系列的烷基胺来源,包括仲烷基胺、伯烷基胺,甚至是空间阻碍较小的伯胺,并证明了与烷基硅烷的高官能团相容性。此外,该反应可用于天然产物和药物的后期修饰,收率高,非对映选择性好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


