Bromotrifluoromethoxylation of allenes: expedient access to allylic trifluoromethoxy derivatives†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d4qo02426e
引用次数: 0
Abstract
A mild and efficient method for the bromotrifluoromethoxylation of allenes has been explored using trifluoromethyl arylsulfonate (TFMS) as the trifluoromethoxy source. A series of allylic trifluoromethoxy derivatives were obtained with high yields and good regioselectivity by terminal selectivity of the allenes. This transition metal-free process expands the scope of the application of trifluoromethoxy reagents.
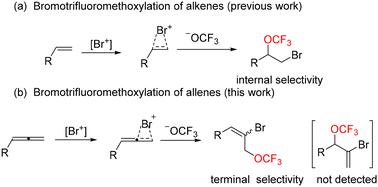
烯基溴三氟甲氧基化:丙烯基三氟甲氧基衍生物的一种权宜之计
以三氟甲基芳基磺酸盐(TFMS)为三氟甲氧基源,探索了一种温和高效的烯基溴三氟甲氧基化反应方法。通过对烯的末端选择性,得到了一系列产率高、区域选择性好的烯丙基三氟甲氧基衍生物。这种过渡无金属工艺扩大了三氟甲氧基试剂的应用范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


