η6-Benzene Tetra-Anion Complexes of Early and Late Rare-Earth Metals
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
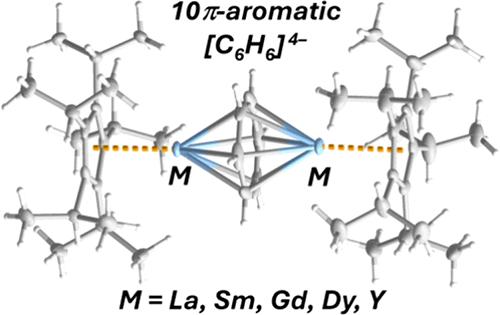
A novel synthetic route to the triple-decker benzene tetra-anion complexes [(η5-C5iPr5)M(μ:η6:η6-C6H6)M(η5-C5iPr5)] is reported for a range of early and late rare-earth elements, i.e., M = Y, La, Sm, Gd, and Dy (1M). The lanthanum complex 1La is the first benzene tetra-anion complex of the largest rare-earth element. Aromaticity in the 10π-electron benzene ligands is confirmed through crystallographic studies of all compounds and nucleus-independent chemical shift calculations on 1Y and 1La. Analysis of the bonding in 1Y and 1La using density functional theory revealed strong covalency in the metal-benzene interactions, with very similar contributions from the metal 4d/5d orbitals, respectively, and the benzene π* orbitals. Magnetic susceptibility measurements on 1Sm, 1Gd, and 1Dy are also consistent with the presence of a benzene tetra-anion ligand. The origins of the appreciable exchange coupling constant of Jexch = −3.35 cm–1 (−2J formalism) in 1Gd are established through a computational study of the interacting magnetic orbitals. The dynamic magnetic properties of 1Dy are also described. The clear absence of SMM behavior in the dysprosium complex is explained using multireference calculations and an ab initio ligand-field theory description of the 4f orbitals, which clearly show that the benzene tetra-anion ligand provides a dominant equatorial contribution.
早期和晚期稀土金属的η - 6-苯四阴离子配合物
以M = Y、La、Sm、Gd、Dy (1M)等早期和晚期稀土元素为原料,提出了一种新的合成三层苯四阴离子配合物[(η5-C5iPr5)M(μ:η6:η6- c6h6)M(η5-C5iPr5)]的新方法。镧配合物1La是最大的稀土元素中第一个苯四阴离子配合物。通过对所有化合物的晶体学研究和对1Y和1La的核无关化学位移计算,证实了10π电子苯配体的芳构性。利用密度泛函理论对1Y和1La的键合进行分析,发现金属-苯相互作用具有很强的共价作用,金属的4d/5d轨道和苯的π*轨道的共价作用非常相似。在1Sm, 1Gd和1Dy上的磁化率测量也与苯四阴离子配体的存在一致。通过对相互作用磁轨道的计算研究,建立了1Gd中Jexch = - 3.35 cm-1 (- 2J形式)的可测交换耦合常数的来源。还描述了1Dy的动态磁性能。用多参考计算和4f轨道的从头算配体场理论解释了镝配合物中SMM行为的明显缺失,这清楚地表明苯四阴离子配体提供了主要的赤道贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


