Electrochemical decarboxylation of medium-chain fatty acids into hydrocarbons controlled by the polymer coatings on carbon-based electrodes†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
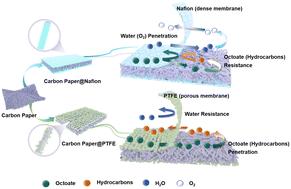
Electrochemical conversion of carboxylic acids for biofuel synthesis is a significant avenue for valorizing biomass. However, the construction of catalyst coatings on electrode surfaces and their effects on the oxidative decarboxylation of medium-chain carboxylic acids remain insufficiently understood. This study examines two types of universal binders, Nafion and PTFE, along with various catalytic materials with decarboxylation activity (such as raw carbon paper (RCP), carbon black (CB), graphite flakes (GFs), carbon nanotubes (CNTs), and Pt/C) for the preparation of catalyst coatings. Through a series of characterization techniques, it was found that coatings containing Nafion exhibit hydrophilic and dense properties, while PTFE-containing coatings display hydrophobic characteristics and a macroporous morphology. Due to these attributes, Nafion coatings are prone to blockage during the electrolysis of medium-chain carboxylic acids (octanoate ions), which inhibits the decarboxylation reaction and favors the occurrence of the oxygen evolution reaction (OER). In contrast, the macroporous structure of PTFE coatings not only facilitates exposure of the catalytic active sites but also hinders the diffusion of water molecules, thereby reducing the OER. This study provides valuable insights and design considerations for the development of highly active electrochemical decarboxylation electrodes.
碳基电极上的聚合物涂层控制中链脂肪酸的电化学脱羧成碳氢化合物
羧酸的电化学转化是生物燃料合成的重要途径。然而,电极表面催化剂涂层的构建及其对中链羧酸氧化脱羧的影响仍未得到充分了解。本研究考察了两种类型的通用粘合剂,Nafion和PTFE,以及各种具有脱羧活性的催化材料(如原碳纸(RCP),炭黑(CB),石墨片(GFs),碳纳米管(CNTs)和Pt/C)用于制备催化剂涂层。通过一系列表征技术,发现含Nafion的涂层具有亲水性和致密性,而含ptfe的涂层具有疏水性和大孔形态。由于这些特性,在电解中链羧酸(辛酸盐离子)时,Nafion涂层容易被堵塞,从而抑制脱羧反应,有利于析氧反应(OER)的发生。相比之下,PTFE涂层的大孔结构不仅有利于催化活性位点的暴露,而且阻碍了水分子的扩散,从而降低了OER。该研究为高活性电化学脱羧电极的开发提供了有价值的见解和设计考虑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


