Coulomb Field-Driven Desorption/Ionization by Femtosecond Laser for Mass Spectrometry Detection and Imaging
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
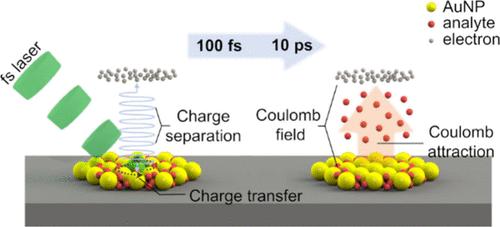
Surface-assisted laser desorption/ionization (SALDI) offers promising prospects for mass spectrometry detection and imaging of small biomolecules, as it addresses most of the matrix-related issues encountered in conventional matrix-assisted laser desorption/ionization (MALDI). Currently, nearly all of the fundamental aspects and applications of SALDI depend on nanosecond (ns) lasers, whereas few efforts have been made to integrate ultrafast femtosecond (fs) lasers with SALDI. Therefore, the intrinsic fundamental principle remains poorly understood. Herein, a novel surface-assisted femtosecond laser desorption/ionization mass spectrometry (fs-SALDI-MS) platform was developed, which significantly reduces analyte fragmentation and preserves molecular integrity. Spectral interferences from surface-assisted materials and alkali-metal adducts are absent in fs-SALDI mass spectra. Ion survival yields continuously increase with decreasing laser pulse widths from 5 ns to 600 fs, highlighting a gradual transition from thermal to nonthermal effects. A lower absolute limit of detection down to ∼3 amol for representative antifungal and psychotropic drugs and clearer visualization of ultratrace drug residues on latent fingerprints can be achieved, indicating that fs-SALDI results in gentler and more efficient detection/ionization processes than mainstream ns-SALDI. The biological applicability of this method was further validated through 10 μm-spatial-resolution lipid imaging of mouse brain sections. In short, a novel Coulomb field-driven desorption/ionization mechanism is proposed for fs-SALDI, opening new avenues for the development of emerging fs-SALDI techniques with superior analytical performance.
飞秒激光库仑场驱动解吸/电离用于质谱检测和成像
表面辅助激光解吸/电离(SALDI)解决了传统基质辅助激光解吸/电离(MALDI)中遇到的大多数与基质相关的问题,为生物小分子的质谱检测和成像提供了广阔的前景。目前,SALDI几乎所有的基本方面和应用都依赖于纳秒(ns)激光器,而将超快飞秒(fs)激光器与SALDI集成的努力却很少。因此,人们对其内在的基本原理仍然知之甚少。本文开发了一种新的表面辅助飞秒激光解吸/电离质谱(fs-SALDI-MS)平台,该平台显著减少了分析物的碎片化并保持了分子完整性。在fs-SALDI质谱中不存在表面辅助材料和碱金属加合物的光谱干扰。随着激光脉冲宽度从5 ns到600 fs的减小,离子存活产率持续增加,突出了从热效应到非热效应的逐渐转变。具有代表性的抗真菌药物和精神药物的检测绝对限低至~ 3 amol,并且可以在潜在指纹上更清晰地显示超痕量药物残留,这表明fs-SALDI的检测/电离过程比主流ns-SALDI更温和,更有效。通过10 μm空间分辨率的小鼠脑切片脂质成像进一步验证了该方法的生物学适用性。总之,提出了一种新的库仑场驱动的fs-SALDI解吸/电离机制,为开发具有优异分析性能的新兴fs-SALDI技术开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


