Electrocatalytic Oxidation of Ammonia to Nitrate Occurs on NiOOH with OH/O Vacancies
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The ammonia electrooxidation reaction (AOR) has attracted significant attention for both wastewater treatment and energy storage applications. However, AOR pathways generating oxygenated products such as nitrite or nitrate remain unresolved. Attenuated total reflection–surface-enhanced infrared absorption spectroscopy (ATR–SEIRAS) and density functional theory (DFT) have been combined to determine the potential-dependent intermediates, active catalytic sites, and AOR pathways on Ni electrodes under alkaline conditions. The OH/O vacancy sites on NiOOH, revealed by XPS, are found by DFT calculations to be the active sites for the AOR, catalyzing the complete (eight-electron) oxidation of ammonia into nitrate. The formation of isolated OH/O vacancies on NiOOH is thermodynamically more favorable than that of paired vacancies in the potential range conducive to NiOOH formation and the AOR, with an increasing formation barrier at higher potentials. This synergistic ATR–SEIRAS and DFT study reveals that the selectivity of nitrite and nitrate production is potential-dependent, with nitrite and hydrazine formation initiated at moderate anodic potentials, whereas larger potentials promote conversion of nitrite into nitrate species. The release of adsorbed nitrite and nitrate, which is crucial for liberating catalytic sites for the continuous AOR, is spectroscopically and computationally shown to be facilitated at relatively low potentials. Comprehending AOR mechanisms on Ni-based catalysts as achieved in this study can pave the way for future research on catalyst design and optimization of AOR performance.
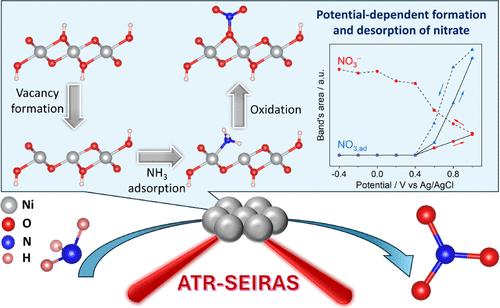
氨在有OH/O空位的NiOOH上电催化氧化生成硝酸盐
氨电氧化反应(AOR)在废水处理和储能方面的应用受到了广泛的关注。然而,产生含氧产物如亚硝酸盐或硝酸盐的AOR途径仍未得到解决。衰减全反射-表面增强红外吸收光谱(ATR-SEIRAS)和密度泛函理论(DFT)相结合,确定了碱性条件下Ni电极上的电位依赖中间体、活性催化位点和AOR途径。通过DFT计算,发现NiOOH上的OH/O空位是AOR的活性位点,催化氨的完全(8电子)氧化成硝酸盐。在有利于NiOOH形成和AOR的电位范围内,NiOOH上孤立OH/O空位的形成在热力学上比成对空位的形成更有利,在高电位下形成势垒增加。这项协同ATR-SEIRAS和DFT研究表明,亚硝酸盐和硝酸盐生成的选择性是电位依赖的,在中等阳极电位下,亚硝酸盐和肼的生成开始,而较大的电位促进亚硝酸盐转化为硝酸盐。吸附的亚硝酸盐和硝酸盐的释放对于释放连续AOR的催化位点至关重要,光谱和计算表明在相对低的电位下更容易释放。本研究对镍基催化剂AOR机理的理解,为进一步研究催化剂的设计和AOR性能的优化奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


