1,1,1,3,3,3-Hexafluoroisopropanol-Promoted Synthesis of Structurally Diverse Alkylidene-4-thiazolidinones/selenazolidinones Involving an Azaoxyallyl Cation
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A one-pot process involving cycloaddition of the azaoxyallyl cation with thioamide and a synchronous E1-type elimination of the C2 amino group from the cycloadduct is disclosed, leading to diverse alkylidene-4-thiazolidinones. Amine elimination under acid-free conditions or without quaternization and forging a stereoselective olefin formation were among the interesting reactivity traits revealed through the present work. Conjugated thioamide permitted side-chain branching through a three-component process. Access to spirooxindolyl thiazolidinones and selenazolidinones adds further value to the process.
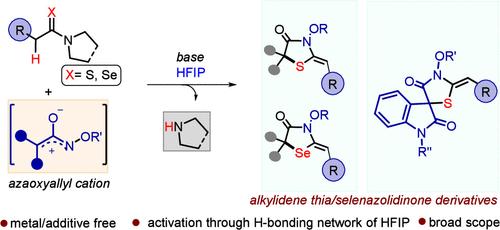
1,1,1,3,3,3-六氟异丙醇促进含氮氧基烯丙基阳离子的结构不同的烷基-4-噻唑烷酮/硒化唑烷酮的合成
本发明公开了一种一锅法,涉及氮氧基烯基阳离子与硫酰胺的环加成以及环加合物中C2氨基的同步e1型消去,从而产生多种烷基烷-4-噻唑烷酮。在无酸条件下或在没有季铵化的情况下,胺消除和形成立体选择性烯烃是本研究揭示的有趣的反应性特征之一。共轭硫酰胺允许侧链分支通过三组分的过程。获得螺新吲哚基噻唑烷酮和硒化噻唑烷酮进一步增加了该过程的价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


