Mechanism and dynamics of photoswitchable flavoprotein charge-transfer complexes
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
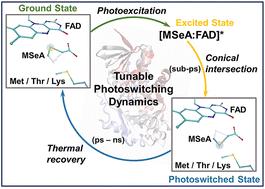
Because of their diverse uses in biological science and engineering, continued effort has been made to expand the pool of photoswitchable protein systems. A recent study demonstrated that in monomeric sarcosine oxidase (MSOX), photoexcitation of a charge-transfer (CT) complex formed by a flavin cofactor and a nonreactive ligand (e.g., methylthioacetate) induces the ligand to reversibly change conformation, with implications for the development of flavin-dependent fast photochromic proteins. However, the factors that control the underlying switching mechanism and dynamics remain largely unexplored. Herein, combining extensive protein mutagenesis, ultrafast laser spectroscopic measurements and classical and quantum computational approaches, we assess those factors in a range of protein variants, including those of MSOX and another flavoenzyme, N-methyltryptophan oxidase (MTOX), where we find that a similar photoswitching cycle can occur. We demonstrate that (1) the kinetic behaviors of the photoswitching cycle are protein- and ligand-dependent; (2) the photoswitching and backward thermal recovery rates can be tuned by mutation of a specific active-site residue (Met245 and Thr239 in MSOX and MTOX, respectively), with recovery rates spanning over an order of magnitude, and (3) modifications of the protein environment alter the conformational energy landscape of the ligand–flavin complex, consequently regulating the photocycle. Taken together, these findings highlight the versatility of such photoswitchable systems, providing a molecular basis for fine-tuning their photophysical properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


