An Adsorption Isotherm That Includes the Interactions between Adsorbates
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
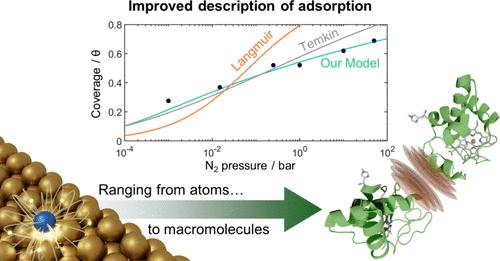
We have developed a new adsorption isotherm that includes repulsive interactions between the adsorbates. By regarding the adsorbate–surface system as a finite array of classical dipoles, we can theoretically derive that the repulsive electrostatic interactions lead to a quadratic coverage dependence in the adsorption energy. On a phenomenological level, density functional theory calculations for N atoms on an Fe{100} surface show that adsorbates interact by a combination of electronic and electrostatic interactions along with positional relaxations to minimize repulsive interactions. We observe that the combination of all these effects also leads to a quadratic coverage dependence in the adsorption energy (or cubic, if the adsorbates cannot relax positionally). The quadratic coverage dependence reflects that the electronic and electrostatic adsorbate interaction mechanisms are highly localized and therefore relatively more important at higher coverages where more adsorbates come into closer proximity. This quadratic coverage dependence is incorporated into the new isotherm. When evaluated against the dissociative adsorption of nitrogen on an industrial iron catalyst for ammonia synthesis, the new isotherm is superior to the classic Frumkin-Temkin isotherms and far superior to the Langmuir isotherm. These new measurements also show that the existing microkinetic models, which constitute the state of the art understanding of industrial ammonia synthesis, overestimate the critical N2 dissociation rate by multiple orders of magnitude due to oversimplifications in the underlying Langmuir descriptions. This illustrates the value of the new isotherm for description of adsorption in connection with catalytic reactions. Additionally, the new isotherm provides a superior description of diverse cases such as nutrient adsorption on soil particles, chromatographic purification of proteins, and adsorption processes for CO2 capture.
包括吸附剂之间相互作用的等温线
我们已经开发了一种新的吸附等温线,包括吸附剂之间的排斥相互作用。通过将吸附表面系统视为经典偶极子的有限阵列,我们可以从理论上推导出斥力静电相互作用导致吸附能的二次覆盖依赖关系。在现象学水平上,对Fe{100}表面上N原子的密度泛函理论计算表明,吸附物通过电子和静电相互作用的组合以及最小化排斥相互作用的位置弛豫来相互作用。我们观察到,所有这些影响的组合也导致吸附能的二次覆盖依赖(或立方,如果吸附物不能放松位置)。二次覆盖依赖反映了电子和静电吸附物相互作用机制是高度局部化的,因此在更高的覆盖下,更多的吸附物更接近,相对更重要。这种二次覆盖依赖关系被纳入新的等温线。当对氨合成用工业铁催化剂上氮的解离吸附进行评价时,新等温线优于经典的Frumkin-Temkin等温线,远优于Langmuir等温线。这些新的测量还表明,现有的微动力学模型,构成了对工业氨合成的最先进的理解,由于在潜在的Langmuir描述中过度简化,高估了几个数量级的临界N2解离率。这说明了新等温线在描述与催化反应有关的吸附方面的价值。此外,新的等温线提供了多种情况的优越描述,如土壤颗粒上的养分吸附,蛋白质的色谱纯化和二氧化碳捕获的吸附过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


