Deciphering the Competitive Charge Storage Chemistry of Metal Cations and Protons in Aqueous MnO2-Based Supercapacitors
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The complex charge storage mechanisms in aqueous MnO2-based supercapacitors have posed significant challenges to a comprehensive understanding of their chemical behavior. In this study, we employed Au-core@MnO2-shell nanoparticle-enhanced Raman spectroscopy, alongside electrochemical analysis and X-ray absorption, to systematically investigate the competitive charge storage chemistry of protons and cations within the inner and outer layers of δ-MnO2 under alkaline conditions. Our findings reveal that δ-MnO2 operates through a dual mechanism: the intercalation and deintercalation of metal cations dominate charge storage in the inner layer, while surface chemisorption of protons governs the outer layer. Notably, cation insertion induces an irreversible phase transition from MnO2 to Mn2O3, whereas the surface redox process involves a reversible transformation among MnO2, MnOOH, and Mn(OH)2. Additionally, spectral evidence, supported by ab initio molecular dynamics simulations, elucidates the structural changes of interfacial water associated with proton-mediated charge storage in the outer layer. Electrochemical analysis further demonstrates that surface charge storage, primarily mediated by a proton-coupled electron transfer mechanism, is the dominant contributor to the overall capacitance. This work not only advances the molecular-level understanding of electrochemical processes in MnO2-based supercapacitors but also highlights the potential for optimizing surface proton-coupled electron transfer mechanisms to enhance capacitive performance.
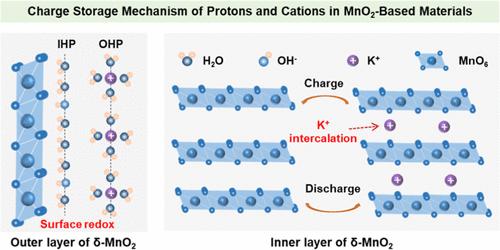
基于二氧化锰的水基超级电容器中复杂的电荷存储机制为全面了解其化学行为带来了巨大挑战。在本研究中,我们采用金核@MnO2-壳纳米粒子增强拉曼光谱以及电化学分析和 X 射线吸收,系统地研究了在碱性条件下,δ-MnO2 内外层质子和阳离子的竞争性电荷存储化学反应。我们的研究结果表明,δ-MnO2 通过双重机制运行:金属阳离子的插层和脱插层主导了内层的电荷存储,而质子的表面化学吸附则主导了外层的电荷存储。值得注意的是,阳离子的插入会引起从 MnO2 到 Mn2O3 的不可逆相变,而表面氧化还原过程则涉及 MnO2、MnOOH 和 Mn(OH)2 之间的可逆转变。此外,光谱证据和 ab initio 分子动力学模拟支持阐明了与质子介导的外层电荷储存有关的界面水的结构变化。电化学分析进一步证明,主要由质子耦合电子转移机制介导的表面电荷存储是总体电容的主要贡献者。这项研究不仅加深了人们对基于二氧化锰的超级电容器中电化学过程的分子层面的理解,而且凸显了优化表面质子耦合电子传递机制以提高电容性能的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


