CDK4 selective inhibition improves preclinical anti-tumor efficacy and safety
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
CDK4/6 inhibitors have revolutionized treatment of hormone receptor positive (HR+), HER2 non-amplified (HER2−) breast cancer. Yet, all “dual” CDK4/6 inhibitors show common dose-limiting hematologic toxicities, foremost neutropenia. This poses challenges to provide these agents at concentrations necessary to extinguish cell cycling in tumors. HR+ breast cancer cells are highly dependent on CDK4 but not CDK6. By contrast, CDK4 is dispensable for human bone marrow derived cells, due to the primary and compensatory role of CDK6 in hematopoiesis. This prompted us to develop atirmociclib (PF-07220060), a next-generation CDK4 selective inhibitor. Atirmociclib’s impact on circulating neutrophils was reduced, in proportion with its increase in CDK4 versus CDK6 selectivity. Realized dose intensification led to greater CDK4 inhibition and deeper anti-tumor responses, pointing to CDK4 target coverage as a limiting factor of CDK4/6 inhibitor efficacy. We also highlight combinatorial agents that may counter acquired resistance to CDK4 selective inhibition and widen its clinical application.
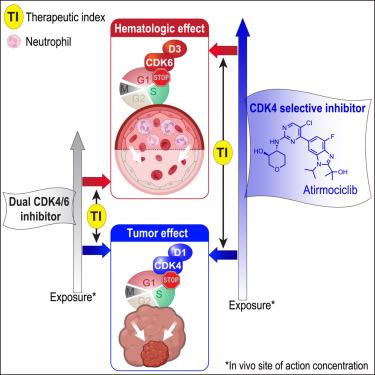
CDK4选择性抑制提高临床前抗肿瘤疗效和安全性
CDK4/6抑制剂已经彻底改变了激素受体阳性(HR+), HER2非扩增(HER2−)乳腺癌的治疗。然而,所有“双重”CDK4/6抑制剂都表现出共同的剂量限制性血液学毒性,主要是中性粒细胞减少。这就提出了在肿瘤中提供必要浓度的这些药物来消灭细胞周期的挑战。HR+乳腺癌细胞高度依赖CDK4而不依赖CDK6。相比之下,由于CDK6在造血中的主要和代偿作用,CDK4对于人类骨髓来源的细胞是必不可少的。这促使我们开发了下一代CDK4选择性抑制剂atirmociclib (PF-07220060)。Atirmociclib对循环中性粒细胞的影响降低,与CDK4选择性与CDK6选择性的增加成比例。实现的剂量增强导致更大的CDK4抑制和更深的抗肿瘤反应,表明CDK4靶点覆盖是CDK4/6抑制剂疗效的限制因素。我们还强调了可能对抗CDK4选择性抑制获得性耐药的组合药物,并扩大了其临床应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


