Low-dose irradiation of the gut improves the efficacy of PD-L1 blockade in metastatic cancer patients
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The mechanisms governing the abscopal effects of local radiotherapy in cancer patients remain an open conundrum. Here, we show that off-target intestinal low-dose irradiation (ILDR) increases the clinical benefits of immune checkpoint inhibitors or chemotherapy in eight retrospective cohorts of cancer patients and in tumor-bearing mice. The abscopal effects of ILDR depend on dosimetry (≥1 and ≤3 Gy) and on the metabolic and immune host-microbiota interaction at baseline allowing CD8+ T cell activation without exhaustion. Various strains of Christensenella minuta selectively boost the anti-cancer efficacy of ILDR and PD-L1 blockade, allowing emigration of intestinal PD-L1-expressing dendritic cells to tumor-draining lymph nodes. An interventional phase 2 study provides the proof-of-concept that ILDR can circumvent resistance to first- or second-line immunotherapy in cancer patients. Prospective clinical trials are warranted to define optimal dosimetry and indications for ILDR to maximize its therapeutic potential.
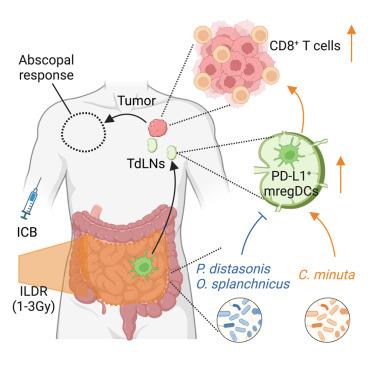
肠道低剂量照射可提高转移性癌症患者PD-L1阻断的疗效
控制局部放疗对癌症患者的体外效应的机制仍然是一个开放的难题。本研究表明,在8组癌症患者和荷瘤小鼠的回顾性队列中,脱靶肠道低剂量照射(ILDR)增加了免疫检查点抑制剂或化疗的临床益处。ILDR的体外效应取决于剂量(≥1 Gy和≤3 Gy)以及代谢和免疫宿主-微生物群在基线时的相互作用,允许CD8+ T细胞激活而不衰竭。不同的分钟克里斯滕森菌菌株选择性地增强了ILDR和PD-L1阻断的抗癌功效,使表达PD-L1的肠道树突状细胞迁移到肿瘤引流淋巴结。一项介入性2期研究提供了概念证明,ILDR可以规避癌症患者对一线或二线免疫治疗的耐药性。有必要进行前瞻性临床试验,以确定ILDR的最佳剂量和适应症,以最大限度地发挥其治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


