Electrochemical N–N Oxidatively Coupled Dehydrogenation of 3,5-Diamino-1H-1,2,4-triazole for Value-Added Chemicals and Bipolar Hydrogen Production
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
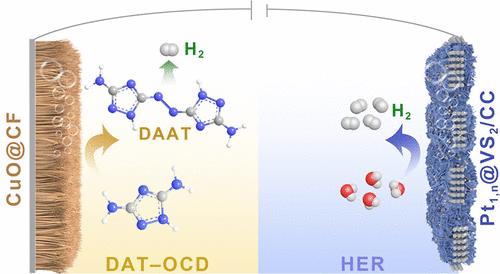
Electrochemical H2 production from water favors low-voltage molecular oxidation to replace the oxygen evolution reaction as an energy-saving and value-added approach. However, there exists a mismatch between the high demand for H2 and slow anodic reactions, restricting practical applications of such hybrid systems. Here, we propose a bipolar H2 production approach, with anodic H2 generation from the N–N oxidatively coupled dehydrogenation (OCD) of 3,5-diamino-1H-1,2,4-triazole (DAT), in addition to the cathodic H2 generation. The system requires relatively low oxidation potentials of 0.872 and 1.108 V vs RHE to reach 10 and 500 mA cm–2, respectively. The bipolar H2 production in an H-type electrolyzer requires only 0.946 and 1.129 V to deliver 10 and 100 mA cm–2, respectively, with the electricity consumption (1.3 kWh per m3 H2) reduced by 68%, compared with conventional water splitting. Moreover, the process is highly appealing due to the absence of traditional hazardous synthetic conditions of azo compounds at the anode and crossover/mixing of H2/O2 in the electrolyzer. A flow-type electrolyzer operates stably at 500 mA cm–2 for 300 h. Mechanistic studies reveal that the Pt single atom and nanoparticle (Pt1,n) optimize the adsorption of the S active sites for H2 production over the Pt1,n@VS2 cathodic catalysts. At the anode, the stepwise dehydrogenation of −NH2 in DAT and then oxidative coupling of −N–N– predominantly form azo compounds while generating H2. The present report paves a new way for atom-economical bipolar H2 production from N–N oxidative coupling of aminotriazole and green electrosynthesis of value-added azo chemicals.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


