Coupled Differential Electrochemical Mass Spectrometry and Surface-Enhanced Infrared Absorption Spectroscopic Studies Unravel the Mechanism of Nitric Oxide Electroreduction on Platinum
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
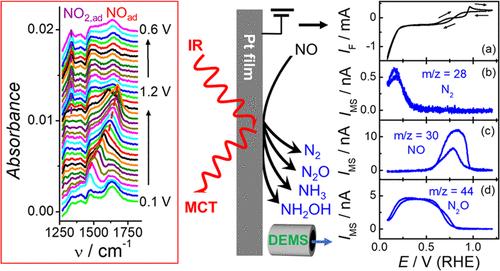
The nitric oxide electroreduction reaction (NORR) has received considerable attention due to its importance in electrochemical denitrification of nitrogen oxides in groundwater and industrial waste gases and electrochemical ammonia synthesis. However, the detailed mechanism and the factors that affect product selectivity are far less understood. Employing coupled differential electrochemical mass spectrometry (DEMS) and attenuated total reflection–surface-enhanced infrared absorption (ATR–SEIRA) spectroscopy, adsorbed species and volatile solution products, during the adsorption of NO and NORR on Pt in both alkaline and acidic media, have been simultaneously studied, enabling us to correlate the potential-dependent product selectivity with the surface ad-species. NOad,M, NOad,B, NOad,L, and NO2,ad were identified using SEIRA spectroscopy as surface ad-species, with their potential-dependent intensities having a strong correlation with the product selectivity. N2O is the only reduction product at potentials beyond the hydrogen region and is attributed to the reduction of weakly adsorbed NO. In contrast, the formation of NH3 and NH2OH occurs only in the hydrogen region and is ascribed to the reaction between strongly adsorbed NO and adsorbed H. N2 is a minor product, and is formed through further reduction of N2O by adsorbed H. The formation of N2 is significantly suppressed in acidic media due to the fast kinetics of NO reduction to NH3/NH2OH, and thus lowering of NO coverage in the hydrogen region. To achieve the selective reduction of NO to NH3/NH2OH, the potential should remain at 0.1–0.2 V (vs RHE) in both acidic and alkaline media while a slow NO supply, and acidic media are preferred over alkaline media due to the faster kinetics. These new spectroscopic results and insights about the NORR could advance the design of more effective NORR catalysts and help develop optimal conditions for selective ammonia synthesis.
耦合差分电化学质谱和表面增强红外吸收光谱研究揭示了氧化氮电还原铂的机理
一氧化氮电还原反应(NORR)在地下水、工业废气中氮氧化物的电化学脱氮和电化学合成氨等方面具有重要意义,受到了广泛的关注。然而,影响产物选择性的具体机理和因素尚不清楚。利用耦合微分电化学质谱(DEMS)和衰减全反射-表面增强红外吸收(ATR-SEIRA)光谱,同时研究了碱性和酸性介质中NO和NORR在Pt上吸附过程中的吸附物质和挥发性溶液产物,使我们能够将电位依赖的产物选择性与表面ad-物质联系起来。利用SEIRA光谱法鉴定出NOad、M、NOad、B、NOad、L和NO2、ad为表面ad物质,它们的电位依赖强度与产物选择性有很强的相关性。N2O是氢区以外电位的唯一还原产物,是由于弱吸附NO的还原。相反,NH3和NH2OH的生成只发生在氢区,是被强吸附的NO和h的反应。N2是次要产物,是被吸附的h进一步还原N2O而形成的。在酸性介质中,由于NO快速还原成NH3/NH2OH的动力学,从而降低了氢区NO的覆盖率,因此N2的形成受到明显抑制。为了实现将NO选择性还原为NH3/NH2OH,在酸性和碱性介质中,当NO供应缓慢时,电位应保持在0.1-0.2 V (vs RHE),并且由于动力学更快,酸性介质优于碱性介质。这些新的光谱结果和关于NORR的见解可以推进更有效的NORR催化剂的设计,并有助于开发选择性氨合成的最佳条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


