Models for T-large granular lymphocytic leukemia: how to mimic the cellular interplays in malignant autoimmunity
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
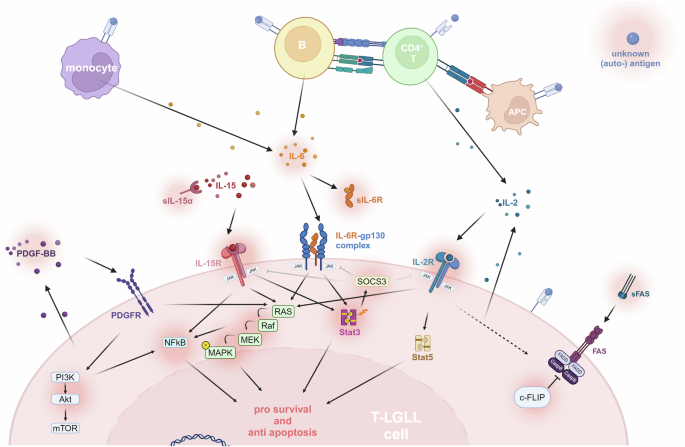
T-large granular lymphocytic leukemia (T-LGLL) is a chronic lymphoproliferative disorder characterized by clonal expansions of cytotoxic T-cells. It presents with cytopenias that are not explained by the typically low leukemic burden. Notably, T-LGLL is frequently accompanied by autoimmune disorders, particularly rheumatoid arthritis (RA). As clonal T-cell expansions are also increasingly identified in autoimmune-driven conditions, better models of T-LGLL’s pathogenesis as a spectrum of (auto)antigen-driven oligoclonal hierarchies towards overt leukemic escape with associated immune dysregulations would provide details to a valuable prototype for determinants of T-cell fitness and transformation as well as T-cell instructed dysfunctions of other immune cells. Such insights would advance our concepts of cancer biology and immunology. Common molecular links between T-LGLL and autoimmune diseases include activation of JAK/STAT signaling, proinflammatory cytokine environments, and antigen-driven immune responses. Current murine models address these mechanisms rather individually: JAK/STAT based systems replicate pathway activation, cytokine-driven models simulate inflammatory conditions, and RA models often mimic antigen stimulation. However, none of these fully captures the duality of clonal T-cell expansion and the complex immune dysregulations, inherent to T-LGLL. This review examines criteria for autochthonous in-vivo T-LGLL models and evaluates existing systems, identifying their strengths, limitations, and specific representations of clinico-pathologic aspects of LGLL. Prominent transgenic models, for example, not only manipulate the T-cell compartment but also indiscriminately alter the tumor microenvironment, impeding research on the specific role of elements of the LGLL micromilieu. We propose strategies to overcome such insufficiencies of present models. Overall, our critical appraisal emphasizes the need for novel comprehensive models that more faithfully integrate the key features of T-LGLL or for models that, by featuring specific pathogenetic aspects of the disease, would supplement existing incomplete systems. We expect such new model systems to aid in better understanding the cancer-immunity interface and in assessing novel therapeutic approaches for T-LGLL.

t大颗粒淋巴细胞白血病模型:如何模拟恶性自身免疫中的细胞相互作用
t大颗粒淋巴细胞白血病(T-LGLL)是一种以细胞毒性t细胞克隆扩增为特征的慢性淋巴细胞增生性疾病。它表现为细胞减少,不能用典型的低白血病负担来解释。值得注意的是,T-LGLL经常伴有自身免疫性疾病,特别是类风湿性关节炎(RA)。由于克隆t细胞扩增也越来越多地在自身免疫驱动的条件下被发现,更好的T-LGLL发病机制模型作为(自身)抗原驱动的寡克隆层次谱系,向明显的白血病逃逸和相关的免疫失调提供细节,这将为t细胞适应性和转化的决定因素以及t细胞指示的其他免疫细胞功能障碍提供有价值的原型。这些见解将推进我们对癌症生物学和免疫学的概念。T-LGLL和自身免疫性疾病之间的常见分子联系包括JAK/STAT信号的激活、促炎细胞因子环境和抗原驱动的免疫反应。目前的小鼠模型相当独立地解决了这些机制:基于JAK/STAT的系统复制通路激活,细胞因子驱动的模型模拟炎症条件,而RA模型通常模拟抗原刺激。然而,这些都没有完全捕捉到克隆t细胞扩增和T-LGLL固有的复杂免疫失调的双重性。本综述考察了自体体内T-LGLL模型的标准,并评估了现有系统,确定了它们的优势、局限性和LGLL临床病理方面的具体表现。例如,突出的转基因模型不仅操纵t细胞区室,而且不加选择地改变肿瘤微环境,阻碍了对LGLL微环境元素具体作用的研究。我们提出了克服现有模型不足的策略。总的来说,我们的批判性评估强调需要新的综合模型,更忠实地整合T-LGLL的关键特征,或者需要通过描述疾病的特定病理方面来补充现有不完整系统的模型。我们期望这样的新模型系统有助于更好地理解癌症-免疫界面,并评估T-LGLL的新治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


