Revealing the Influence of Binding Motifs on Electron Transfer and Recombination Kinetics for CdSe Quantum Dots Functionalized with a Modified Viologen
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
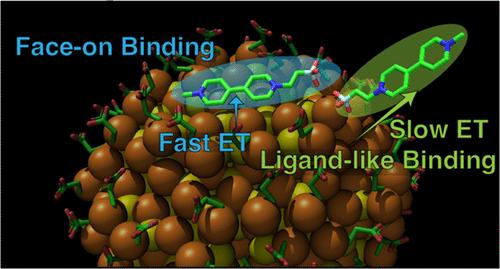
Anchoring of molecules to the surfaces of semiconductor nanocrystals (NCs) presents an opportunity to leverage the precise synthetic tunability of molecular function and the remarkable light harvesting properties of NCs to drive photochemical reactions. However, charge transfer between the two species depends not only on the energy level alignments but also on the details of their binding interactions, which are difficult to probe. Here, we characterize the binding between CdSe quantum dots (QDs) and a new phosphonated derivative of the electron acceptor methyl viologen, designed to attach to the QD surface via the phosphonate group. We use isothermal titration calorimetry to probe the thermodynamics of the QD–molecule interaction and use the parameters determined therein to analyze transient absorption spectroscopy measurements of forward and back electron transfer from QDs to the viologen. We find that the ligand-like phosphonate binding leads to an electron-transfer rate constant that is 3 orders of magnitude smaller than that for the face-on binding of the bipyridine ring of methyl viologen. Back electron transfer is also significantly slower in the derivative. Interestingly, a minor fraction of the phosphonated derivative also binds in the face-on configuration, with similar forward and back electron transfer kinetics as methyl viologen. Numerical simulations show that the ligand-like binding will lead to significantly improved quantum yields of photocatalysis over a wide range of reaction rates. By independently characterizing binding thermodynamics and charge transfer kinetics, this work reveals how the complexities underlying electron transfer at the NC–molecule interface determine photocatalytic outcomes. This work also represents a step toward controlling forward and back electron transfer kinetics via rational molecular design.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


