Immunodrug Delivery by Self-Accelerating Degradable Polycarbonate Micelles with Transiently Stable Hemiacetal Ester Side Chains
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
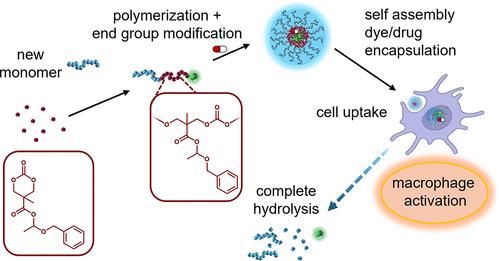
Despite the abundance of established micellar materials for (immuno)drug delivery, they often lack degradability, which is considered a significant aspect toward clinical translation. In this study, we introduce amphiphilic polycarbonates with transiently stable hemiacetal ester side chains, providing a promising degradable alternative to traditional ester side chains but with unique degradation profiles. Cyclic aliphatic carbonate monomers with hemiacetal ester groups can undergo ring-opening polymerization under base catalysis initiated by pyrene butanol or mPEG5k–OH. In water, the resulting block copolymers efficiently self-assemble into polymeric micelles. Initial evidence from NMR and dynamic light scattering (DLS) studies suggests that the hemiacetal ester groups are likely to degrade upon exposure to aqueous solutions, however, remaining significantly stable within the micellar core. We hypothesize that this “on–off” degradation behavior is advantageous, as it reduces potential off-target interactions of liberated amphiphilic molecules with biological species. Additionally, the gained hydrophilicity resulting from side-chain degradation facilitates the breakdown of the polycarbonate backbone compared with aliphatic polycarbonates with hydrophobic ester side groups. Moreover, the polymer end groups can be converted to pentafluorophenyl carbonates for covalent dye labeling. Flow cytometry and microscopy experiments confirm micellar cell uptake and the successful codelivery of covalently attached and hydrophobically encapsulated dyes. Additionally, the micelles effectively solubilize the immunostimulatory drug CL075, mitigating its concentration-dependent toxicity. Our results suggest that self-accelerating degradable polycarbonate micelles derived from transiently stable hemiacetal ester side chains can be applied as a valuable nanosized immunodrug delivery platform.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


