Theoretical Modeling of Direct Z-Scheme B,F-Doped g-C3N4/CoN4 Composites for Promoting Photocatalytic Water Splitting Reaction
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The Z-scheme mechanism bestows a perfect band alignment for photocatalysis due to the high separation efficiency of the photoexcited electron–hole pair and concomitantly preserves the redox ability toward reduction of electrons and oxidation of holes. However, the challenge of modeling and building of Z-scheme photocatalysts hinges on the correct band alignment and the direction of the inherent electric field. Keeping the efficacy of the advanced metaGGA functional to illustrate the band gap of layered materials in mind, we model a Z-scheme photocatalytic mechanism using density functional theory calculations using R2SCAN method. The type I g-C3N4/CoN4 heterojunction with limited photocatalytic activity is switched into a direct Z-scheme configuration through boron and F doping in g-C3N4 and enhances the utilization ratio of visible light for the g-C3N4/CoN4 photocatalyst. The built-in electric field in the B,F-g-C3N4/CoN4 nanocomposite facilitates interface charge transfer and forbids the rapid recombination of photoinduced carriers, causing the B,F-g-C3N4 monolayer with a negative charge and the CoN4 (111) surface with a positive charge. The higher band edge potentials of CoN4(111) surface compared to that of the B,F-g-C3N4 monolayer ensure a stronger redox ability and the built-in electric field along with satisfy the essentiality of B,F-g-C3N4/CoN4 nanocomposite to be a typical Z-scheme heterostructure. In the presence of visible light irradiation, electrons are excited to the conduction band minimum (CBM) of the CoN4 (111) surface enduring the hydrogen evolution reaction (HER), and the holes remain in the valence band maximum (VBM) of the B,F-g-C3N4 monolayer ministering the oxygen evolution reaction (OER). Investigation on the absorption spectra further manifests a large enhancement of the visible light absorption efficiency after boron and fluorine doping. A systematic theoretical analysis of electronic and optical properties, charge transfer, differential charge density distribution, work function, and photocatalytic mechanisms manifests that B,F-g-C3N4/CoN4 is a potential Z-scheme photocatalyst for water splitting reaction under visible light.
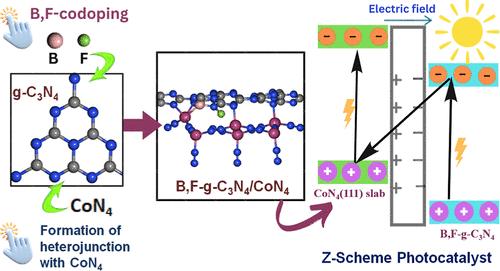
直接Z-Scheme B, f掺杂g-C3N4/CoN4复合材料促进光催化水裂解反应的理论建模
由于光激发电子-空穴对的高分离效率,Z-scheme机制为光催化提供了完美的能带对准,并同时保留了电子还原和空穴氧化的氧化还原能力。然而,z型光催化剂的建模和构建的挑战在于正确的带对准和固有电场的方向。考虑到先进的metaGGA功能的有效性来说明层状材料的带隙,我们使用R2SCAN方法使用密度泛函理论计算来模拟z方案光催化机制。通过在g-C3N4中掺杂硼和F,将光催化活性有限的I型g-C3N4/CoN4异质结直接转变为z型构型,提高了g-C3N4/CoN4光催化剂的可见光利用率。B,F-g-C3N4/CoN4纳米复合材料中内置的电场有利于界面电荷转移,阻止光诱导载流子的快速重组,导致B,F-g-C3N4单层带负电荷,CoN4(111)表面带正电荷。与B,F-g-C3N4单层相比,CoN4(111)表面较高的带边电位保证了其更强的氧化还原能力和内置电场,同时满足了B,F-g-C3N4/CoN4纳米复合材料作为典型z型异质结构的本质。在可见光照射下,电子被激发到CoN4(111)表面的最小导带(CBM),进行析氢反应(HER),空穴留在B,F-g-C3N4单层的价带最大值(VBM),进行析氧反应(OER)。对吸收光谱的研究进一步表明,硼和氟掺杂后可见光吸收效率大大提高。通过对B,F-g-C3N4/CoN4的电子和光学性质、电荷转移、电荷密度分布、功函数和光催化机理的系统理论分析表明,B,F-g-C3N4/CoN4是一种潜在的可见光下水裂解反应的z方案光催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


